Quest for the right Drug
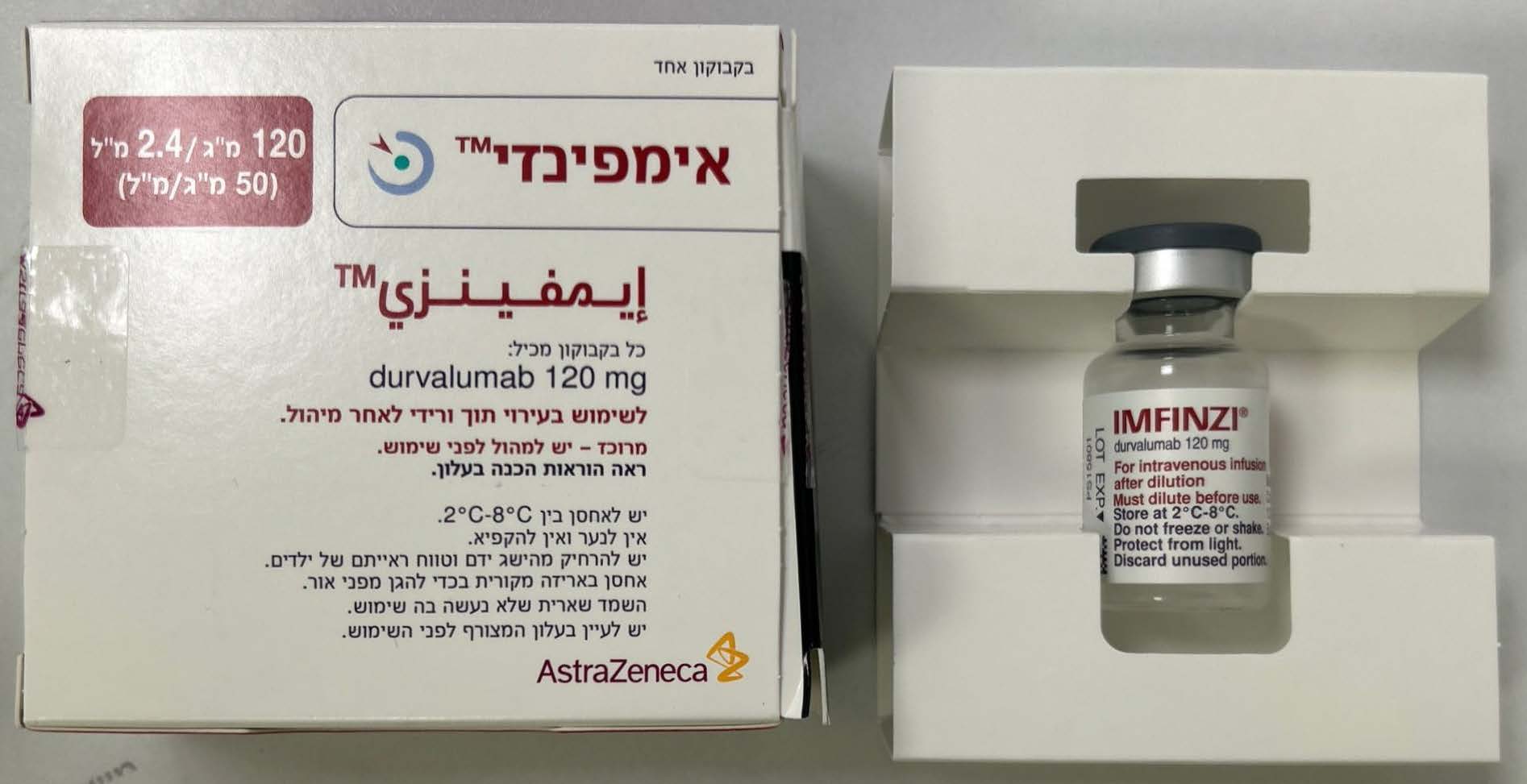
אימפינזי IMFINZI (DURVALUMAB)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
תמיסה לאינפוזיה : SOLUTION FOR INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
6 ADVERSE REACTIONS The following adverse reactions are discussed in greater detail in other sections of the labeling. • Immune-Mediated Adverse Reactions [see Warnings and Precautions (5.1)]. • Infusion-Related Reactions [see Warnings and Precautions (5.2)]. 6.1 Clinical Trials Experience Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The data described in the Warnings and Precautions section reflect exposure to IMFINZI as a single agent in a total of 1889 patients enrolled in the PACIFIC study (a randomized, placebo-controlled study that enrolled 475 patients with Stage III NSCLC), Study 1108 (an open-label, single-arm, multicohort study that enrolled 970 patients with advanced solid tumors), and an additional open-label, single-arm trial (ATLANTIC Study) that enrolled 444 patients with advanced solid tumors, including NSCLC. In these trials, IMFINZI was administered at a dose of 10 mg/kg every 2 weeks. Among the 1889 patients, 38% were exposed for 6 months or more and 18% were exposed for 12 months or more. The data also reflect exposure to IMFINZI in combination with chemotherapy in 265 patients from the CASPIAN study (a randomized, open-label study in patients with ES-SCLC), in 338 patients from the TOPAZ-1 study (a randomized, double-blind study in patients with BTC). In the CASPIAN and TOPAZ-1 studies, IMFINZI was administered at a dose of 1,500 mg every 3 or 4 weeks. The data described in the Warnings and Precautions also reflect exposure to IMFINZI 1,500 mg in combination with tremelimumab 300 mg in 388 patients in HIMALAYA. In the HIMALAYA study patients received IMFINZI 1,500 mg in combination with tremelimumab as a single intravenous infusion of 300 mg, followed by IMFINZI 1,500 mg every 4 weeks. The pooled safety population (N=596) described in the Warnings and Precautions section reflect exposure to IMFINZI 1,500 mg in combination with tremelimumab 75 mg and histology-based platinum chemotherapy regimens in 330 patients in POSEIDON [see Clinical Studies (14.2)] and 266 patients with ES-SCLC in CASPIAN who received up to four cycles of platinum-etoposide plus IMFINZI 1,500 mg with tremelimumab 75 mg every 3 weeks followed by IMFINZI 1,500 mg every 4 weeks (an unapproved regimen for extensive stage small cell lung cancer). Among the 596 patients, 55% were exposed to IMFINZI for 6 months or more and 24% were exposed for 12 months or more. The data described in this section reflect exposure to IMFINZI in patients with Stage III NSCLC enrolled in the PACIFIC study, in patients with metastatic NSCLC enrolled in the POSEIDON study, in patients with ES-SCLC enrolled in the CASPIAN study, in patients with BTC enrolled in the TOPAZ-1 study and in patients with uHCC included in the HIMALAYA study. Urothelial Carcinoma The safety of IMFINZI was evaluated in 182 patients with locally advanced or metastatic urothelial carcinoma in the urothelial carcinoma cohort of Study 1108 whose disease has progressed during or after one standard platinum-based regimen. Patients received 10 mg/kg intravenously every 2 weeks [see Clinical Studies (14.1)]. The median duration of exposure was 2.3 months (range: 1 day to 12.1 months). Thirty-one percent (31%) of patients had a drug delay or interruption for an adverse reaction. The most common (>2%) were liver injury (4.9%), urinary tract infection (3.3%), acute kidney injury (3.3%), and musculoskeletal pain (2.7%). The most common adverse reactions (≥15%) were fatigue (39%), musculoskeletal pain (24%), constipation (21%), decreased appetite (19%), nausea (16%), peripheral edema (15%) and urinary tract infection (15%). The most common Grade 3 or 4 adverse reactions (≥3%) were fatigue, urinary tract infection, musculoskeletal pain, abdominal pain, dehydration, and general physical health deterioration. Eight patients (4.4%) who were treated with IMFINZI experienced Grade 5 adverse events of cardiorespiratory arrest, general physical health deterioration, sepsis, ileus, pneumonitis, or immune- mediated hepatitis. Three additional patients were experiencing infection and disease progression at the time of death. IMFINZI was discontinued for adverse reactions in 3.3% of patients. Serious adverse reactions occurred in 46% of patients. The most frequent serious adverse reactions (>2%) were acute kidney injury (4.9%), urinary tract infection (4.4%), musculoskeletal pain (4.4%), liver injury (3.3%), general physical health deterioration (3.3%), sepsis, abdominal pain, pyrexia/tumor associated fever (2.7% each). Table 5 summarizes the adverse reactions that occurred in ≥10% of patients, while Table 4 summarizes the Grade 3 - 4 laboratory abnormalities that occurred in ≥1% of patients treated with IMFINZI in the urothelial carcinoma cohort of Study 1108. Table 5. Adverse Reactions in ≥10% of Patients in study 1108 Urothelial Carcinoma Cohort IMFINZI (N=182) Adverse Reaction All Grades Grades 3 – 4 (%) (%) Gastrointestinal Disorders Constipation 21 1.1 Nausea 16 1.6 Abdominal pain1 14 2.7 Diarrhea/Colitis 13 1.1 General Disorders and Administration Fatigue2 39 6 Peripheral edema3 15 1.6 Pyrexia/Tumor associated fever 14 0.5 Infections Urinary tract infection4 15 4.4 Metabolism and Nutrition Disorders Decreased appetite/Hypophagia 19 0.5 Musculoskeletal and Connective Tissue Disorders Musculoskeletal pain5 24 3.8 Respiratory, Thoracic, and Mediastinal Disorders Dyspnea/Exertional Dyspnea 13 2.2 Cough/Productive Cough 10 0 Skin and Subcutaneous Tissue Disorders Rash6 11 0.5 1 Includes abdominal pain upper, abdominal pain lower and flank pain 2 Includes asthenia, lethargy, and malaise 3 Includes edema, localized edema, edema peripheral, lymphedema, peripheral swelling, scrotal edema, and scrotal swelling 4 Includes cystitis, candiduria and urosepsis 5 Includes back pain, musculoskeletal chest pain, musculoskeletal pain and discomfort, myalgia, and neck pain 6 Includes dermatitis, dermatitis acneiform, dermatitis psoriasiform, psoriasis, rash maculo-papular, rash pruritic, rash papular, rash pustular, skin toxicity, eczema, erythema, erythema multiforme, rash erythematous, acne, and lichen planus Table 6. Grade 3-4 Laboratory Abnormalities Worsened from Baseline Occurring in ≥1% Patients in Study 1108 Urothelial Carcinoma Cohort Laboratory Abnormality Grade 3 – 4 % Hyponatremia 12 Lymphopenia 11 Anemia 8 Increased alkaline phosphatase 4.1 Hypermagnesemia 4.2 Hypercalcemia 3 Hyperglycemia 3 Increased AST 2.4 Increased ALT 0.6 Hyperbilirubinemia 1.2 Increased creatinine 1.2 Neutropenia 1.2 Hyperkalemia 1.2 Hypokalemia 1.2 Hypoalbuminemia 1.2 Non-Small Cell Lung Cancer Stage III NSCLC - PACIFIC The safety of IMFINZI in patients with Stage III NSCLC who completed concurrent platinum-based chemoradiotherapy within 42 days prior to initiation of study drug was evaluated in the PACIFIC study, a multicenter, randomized, double-blind, placebo-controlled study. A total of 475 patients received IMFINZI 10 mg/kg intravenously every 2 weeks. The study excluded patients who had disease progression following chemoradiation, with active or prior autoimmune disease within 2 years of initiation of the study or with medical conditions that required systemic immunosuppression. [see Clinical Studies (14.2)]. The study population characteristics were: median age of 64 years (range: 23 to 90), 45% age 65 years or older, 70% male, 69% White, 27% Asian, 75% former smoker, 16% current smoker, and 51% had WHO performance status of 1. All patients received definitive radiotherapy as per protocol, of which 92% received a total radiation dose of 54 Gy to 66 Gy. The median duration of exposure to IMFINZI was 10 months (range: 0.2 to 12.6). IMFINZI was discontinued due to adverse reactions in 15% of patients. The most common adverse reactions leading to IMFINZI discontinuation were pneumonitis or radiation pneumonitis in 6% of patients. Serious adverse reactions occurred in 29% of patients receiving IMFINZI. The most frequent serious adverse reactions reported in at least 2% of patients were pneumonitis or radiation pneumonitis (7%) and pneumonia (6%). Fatal pneumonitis or radiation pneumonitis and fatal pneumonia occurred in < 2% of patients and were similar across arms. The most common adverse reactions (occurring in ≥ 20% of patients) were cough, fatigue, pneumonitis or radiation pneumonitis, upper respiratory tract infections, dyspnea and rash. Table 7 summarizes the adverse reactions that occurred in at least 10% of patients treated with IMFINZI. Table 7. Adverse Reactions Occurring in ≥ 10% Patients in the PACIFIC Study IMFINZI Placebo N=475 N=234 Adverse Reaction All Grades Grades 3-4 (%) All Grades (%) Grades (%) 3-4 (%) Respiratory, Thoracic and Mediastinal Disorders Cough/Productive Cough 40 0.6 30 0.4 Pneumonitis2/Radiation Pneumonitis 34 3.4 25 3 Dyspnea3 25 1.5 25 2.6 Gastrointestinal Disorders Diarrhea 18 0.6 19 1.3 Abdominal pain4 10 0.4 6 0.4 Endocrine Disorders Hypothyroidism5 12 0.2 1.7 0 Skin and Subcutaneous Tissue Disorders Rash6 23 0.6 12 0 Pruritus7 12 0 6 0 General Disorders Fatigue8 34 0.8 32 1.3 Pyrexia 15 0.2 9 0 Infections Upper respiratory tract 26 0.4 19 0 infections9 Pneumonia10 17 7 12 6 2 includes acute interstitial pneumonitis, interstitial lung disease, pneumonitis, pulmonary fibrosis 3 includes dyspnea and exertional dyspnea 4 includes abdominal pain, abdominal pain lower, abdominal pain upper, and flank pain 5 includes autoimmune hypothyroidism and hypothyroidism 6 includes rash erythematous, rash generalized, rash macular, rash maculopapular, rash papular, rash pruritic, rash pustular, erythema, eczema, rash and dermatitis 7 includes pruritus generalized and pruritus 8 includes asthenia and fatigue 9 includes laryngitis, nasopharyngitis, peritonsillar abscess, pharyngitis, rhinitis, sinusitis, tonsillitis, tracheobronchitis, and upper respiratory tract infection 10 includes lung infection, pneumocystis jirovecii pneumonia, pneumonia, pneumonia adenoviral, pneumonia bacterial, pneumonia cytomegaloviral, pneumonia haemophilus, pneumonia klebsiella, pneumonia necrotising, pneumonia pneumococcal, and pneumonia streptococcal Other adverse reactions occurring in less than 10% of patients treated with IMFINZI were dysphonia, dysuria, night sweats, peripheral edema, and increased susceptibility to infections. Table 8 summarizes the laboratory abnormalities that occurred in at least 20% of patients treated with IMFINZI. Table 8. Laboratory Abnormalities Worsening From Baseline Occurring in ≥ 20% of Patients in the PACIFIC Study IMFINZI Placeb o Laboratory Abnormality Grade 3 or Grade 3 or All All 4 (%) 4 (%) Grades1 Grades1 (%)2 (%)2 Chemistry Hyperglycemia 52 8 51 8 Hypocalcemia 46 0.2 41 0 Increased ALT 39 2.3 22 0.4 Increased AST 36 2.8 21 0.4 Hyponatremia 33 3.6 30 3.1 Hyperkalemia 32 1.1 29 1.8 Increased GGT 24 3.4 22 1.7 Hematology Lymphopenia 43 17 39 18 1 Graded according to NCI CTCAE version 4.0 2 Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: IMFINZI (range: 464 to 470) and placebo (range: 224 to 228) Metastatic NSCLC - POSEIDON The safety of IMFINZI in combination with tremelimumab and platinum-based chemotherapy in patients with metastatic NSCLC was evaluated in POSEIDON (NCT03164616), a randomized, open-label, multicenter, active-controlled trial. A total of 330 patients received IMFINZI 1,500 mg in combination with tremelimumab (≥ 30 kg body weight received 75 mg and < 30 kg body weight received 1 mg/kg) and histology-based platinum chemotherapy regimens [see Clinical Studies (14.2)]. Of these patients, 66% received the maximum 5 doses of tremelimumab and 79% received at least 4 doses. Treatment was continued with IMFINZI as a single agent (or with IMFINZI and histologically-based pemetrexed for non-squamous patients based on the investigator’s decision) until disease progression or unacceptable toxicity. The trial excluded patients with active or prior autoimmune disease or with medical conditions that required systemic corticosteroids or immunosuppressants [see Clinical Studies (14.2)]. The median age of patients who received IMFINZI in combination with tremelimumab and platinum-based chemotherapy was 63 years (range: 27 to 87); 80% male; 61% White, 29% Asian, 58% former smoker, 25% current smoker, and 68% ECOG performance of 1. Serious adverse reactions occurred in 44% of patients receiving IMFINZI in combination with tremelimumab and platinum-based chemotherapy. The most frequent serious adverse reactions reported in at least 2% of patients were pneumonia (11%), anemia (5%), diarrhea (2.4%), thrombocytopenia (2.4%), pyrexia (2.4%), and febrile neutropenia (2.1%). Fatal adverse reactions occurred in a total of 4.2% of patients receiving IMFINZI in combination with tremelimumab and platinum-based chemotherapy. These include hepatitis, nephritis, myocarditis, pancreatitis (all in the same patient), death (2 patients), sepsis (2 patients), pneumonitis (2 patients), acute kidney injury (2 patients), febrile neutropenia (1 patient), chronic obstructive pulmonary disease (1 patient), dyspnea (1 patient), sudden death (1 patient), and ischemic stroke (1 patient). Permanent discontinuation of IMFINZI or tremelimumab due to an adverse reaction occurred in 17% of the patients. Adverse reactions which resulted in permanent discontinuation of IMFINZI or tremelimumab in > 2% of patients included pneumonia. Dosage interruption or delay of IMFINZI and tremelimumab due to an adverse reaction occurred in 41% of patients. Adverse reactions which required dosage interruption or delay of IMFINZI and tremelimumab in > 1% of patients included anemia, leukopenia/white blood cell count decreased, pneumonia, pneumonitis, colitis, diarrhea, hepatitis, rash, asthenia, amylase increased, alanine aminotransferase increased, aspartate aminotransferase increased, lipase increased, neutropenia/neutrophil count decreased, and thrombocytopenia/platelet count decreased. The most common adverse reactions (occurring in ≥ 20% of patients) were nausea, fatigue, musculoskeletal pain, decreased appetite, rash, and diarrhea. Grade 3 or 4 laboratory abnormalities (≥ 10%) were neutropenia, anemia, leukopenia, lymphocytopenia, lipase increased, hyponatremia and thrombocytopenia. Table 9 summarizes the adverse reactions in POSEIDON. Table 9. Adverse Reactions ( ≥ 10%) in Patients with NSCLC Who Received IMFINZI in the POSEIDON Study IMFINZI with tremelimumab and Platinum-based chemotherapy platinum-based chemotherapy N = 333 N = 330 Adverse Reaction All Grades (%) Grade 3 or 4 (%) All Grades (%) Grade 3 or 4 (%) Respiratory, thoracic and mediastinal disorders IMFINZI with tremelimumab and Platinum-based chemotherapy platinum-based chemotherapy N = 333 N = 330 Adverse Reaction All Grades (%) Grade 3 or 4 (%) All Grades (%) Grade 3 or 4 (%) Cough/Productive Cough* 12 0 8 0.3 Gastrointestinal disorders Nausea 42 1.8 37 2.1 Diarrhea 22 1.5 15 1.5 Constipation 19 0 24 0.6 Vomiting 18 1.2 14 1.5 Stomatitis† 10 0 6 0.3 Endocrine disorders Hypothyroidism‡ 13 0 2.1 0 Skin and subcutaneous tissue disorders Rash§ 27 2.4 10 0.6 Alopecia 10 0 6 0 Pruritus 11 0 4.5 0 General disorders and administration site conditions Fatigue/Asthenia¶ 36 5 32 4.5 Pyrexia# 19 0 8 0 EdemaÞ 10 0 10 0.6 Musculoskeletal and connective tissue disorders Musculoskeletal Painß 29 0.6 22 1.5 Metabolism and nutrition disorders Decreased appetite 28 1.5 25 1.2 Infections and Infestations Pneumonia à 17 8 12 4.2 Upper respiratory tract 15 0.6 9 0.9 infectionsè Nervous system disorders Headacheð 11 0 8 0.6 * Includescough and productive cough. † Includes mucosal inflammation and stomatitis. ‡ Includes blood thyroid stimulating hormone increased and hypothyroidism. § Includes eczema, erythema, dermatitis, drug eruption, erythema multiforme, pemphigoid, rash, rash maculo-papular, rash papular, rash pruritic, and rash pustular. ¶ Includes asthenia and fatigue. # Includes body temperature increased, hyperpyrexia, hyperthermia, and pyrexia. Þ Includes face edema, localized edema, and edema peripheral. ß Includes arthralgia, arthritis, back pain, bone pain, musculoskeletal chest pain, musculoskeletal pain, myalgia, neck pain, non- cardiac chest pain, spinal pain. à Includes lower respiratory tract infection, pneumocystis jirovecii pneumonia, pneumonia, pneumonia aspiration, pneumonia bacterial. è Includes laryngitis, nasopharyngitis, pharyngitis, rhinitis, sinusitis, tonsillitis, tracheobronchitis and upper respiratory tract infection. ð Includes headache, migraine. Table 10 summarizes the laboratory abnormalities in POSEIDON. Table 10. Select Laboratory Abnormalities (≥ 10%) That Worsened from Baseline in Patients with NSCLC Who Received IMFINZI in the POSEIDON Study Laboratory Abnormality* IMFINZI with tremelimumab and Platinum-based chemotherapy§ platinum-based chemotherapy† All Grades Grade 3 or 4 All Grades Grade 3 or 4 (%) (%) (%) (%) Chemistry Lipase increased 35 14 25 5 Hyponatremia 55 13 50 11 Hypernatremia 15 0 14 0 Amylase increased 41 9 25 6 Hypokalemia 21 7 17 2.8 Hyperglycemia 42 6 37 3.1 Increased ALT 64 6 56 4.7 Increased AST 63 5 55 2.2 Blood creatinine increased 89 4.0 83 1.9 Increased Alkaline 33 3.4 26 1.2 Phosphatase Gamma Glutamyl 38 2.2 35 4.7 Transferase increased Hyperkalemia 49 2.2 35 2.8 Albumin decreased 27 1.9 18 0.9 Hypocalcemia 58 0.9 49 0.9 Hypomagnesemia 12 4 23 0 Laboratory Abnormality* IMFINZI with tremelimumab and Platinum-based chemotherapy§ platinum-based chemotherapy† All Grades Grade 3 or 4 All Grades Grade 3 or 4 (%) (%) (%) (%) Bilirubinemia 16 0.9 8 0.3 Hematology Neutropenia 71 37 69 32 Anemia 84 24 84 25 Leukopenia 77 21 81 18 Lymphocytopenia 67 20 60 19 Thrombocytopenia 53 11 54 12 * Graded according to NCI CTCAE version 4.03. † The denominator used to calculate the rate varied from 45 to 326 based on the number of patients with a baseline value and at least one post-treatment value. § The denominator used to calculate the rate varied from 43 to 323 based on the number of patients with a baseline value and at least one post-treatment value. Small Cell Lung Cancer Extensive Stage Small Cell Lung Cancer – CASPIAN The safety of IMFINZI in combination with etoposide and either carboplatin or cisplatin in previously untreated ES-SCLC was evaluated in CASPIAN, a randomized, open-label, multicenter, active-controlled trial. A total of 265 patients received IMFINZI 1,500 mg in combination with chemotherapy every 3 weeks for 4 cycles followed by IMFINZI 1,500 mg every 4 weeks until disease progression or unacceptable toxicity. The trial excluded patients with active or prior autoimmune disease or with medical conditions that required systemic corticosteroids or immunosuppressants [see Clinical Studies (14.3)]. Among 265 patients receiving IMFINZI, 49% were exposed for 6 months or longer and 19% were exposed for 12 months or longer. Among 266 patients receiving chemotherapy alone, 57% of the patients received 6 cycles of chemotherapy and 8% of the patients received prophylactic cranial irradiation (PCI) after chemotherapy. IMFINZI was discontinued due to adverse reactions in 7% of the patients receiving IMFINZI plus chemotherapy. These include pneumonitis, hepatotoxicity, neurotoxicity, sepsis, diabetic ketoacidosis and pancytopenia (1 patient each). Serious adverse reactions occurred in 31% of patients receiving IMFINZI plus chemotherapy. The most frequent serious adverse reactions reported in at least 1% of patients were febrile neutropenia (4.5%), pneumonia (2.3%), anemia (1.9%), pancytopenia (1.5%), pneumonitis (1.1%) and COPD (1.1%). Fatal adverse reactions occurred in 4.9% of patients receiving IMFINZI plus chemotherapy. These include pancytopenia, sepsis, septic shock, pulmonary artery thrombosis, pulmonary embolism, and hepatitis (1 patient each) and sudden death (2 patients). The most common adverse reactions (occurring in ≥ 20% of patients) were nausea, fatigue/asthenia and alopecia. Table 11 summarizes the adverse reactions that occurred in patients treated with IMFINZI plus chemotherapy. Table 11. Adverse Reactions Occurring in ≥ 10% of Patients in the CASPIAN study IMFINZI with etoposide and Etoposide and either either carboplatin or cisplatin carboplatin or cisplatin N = N = 265 266 Adverse Reaction All Grades (%) Grade 3-4 (%) All Grades (%) Grade 3-4 (%) Respiratory, thoracic and mediastinal disorders Cough/Productive 15 0.8 9 0 Cough Gastrointestinal disorders Nausea 34 0.4 34 1.9 Constipation 17 0.8 19 0 Vomiting 15 0 17 1.1 Diarrhea 10 1.1 11 1.1 Endocrine disorders Hyperthyroidisma 10 0 0.4 0 Skin and subcutaneous tissue disorders Alopecia 31 1.1 34 0.8 Rashb 11 0 6 0 General disorders and administration site conditions Fatigue/Asthenia 32 3.4 32 2.3 Metabolism and nutrition disorders Decreased appetite 18 0.8 17 0.8 a Includes hyperthyroidism and Basedow's disease b Includes rash erythematous, rash generalized, rash macular, rash maculopapular, rash papular, rash pruritic, rash pustular, erythema, eczema, rash and dermatitis Table 12 summarizes the laboratory abnormalities that occurred in at least 20% of patients treated with IMFINZI plus chemotherapy. Table 12. Laboratory Abnormalities Worsening from Baseline Occurring in ≥ 20%1 of Patients in the CASPIAN study IMFINZI with Etoposide and Etoposide and either either Carboplatin Carboplatin or or Cisplatin Cisplatin Laboratory Abnormality Grade2 3 or 4 (%)3 Grade2 3 or 4 (%)3 Chemistry Hyponatremia 11 13 Hypomagnesemia 11 6 Hyperglycemia 5 5 Increased Alkaline Phosphatase 4.9 3.5 Increased ALT 4.9 2.7 Increased AST 4.6 1.2 Hypocalcemia 3.5 2.4 Blood creatinine increased 3.4 1.1 Hyperkalemia 1.5 3.1 TSH decreased < LLN4 and ≥ LLN at NA NA baseline Hematology Neutropenia 41 48 Lymphopenia 14 13 Anemia 13 22 Thrombocytopenia 12 15 1 The frequency cut off is based on any grade change from baseline 2 Graded according to NCI CTCAE version 4.03 3 Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: IMFINZI (range: 258 to 263) and chemotherapy (range: 253 to 262) except magnesium IMFINZI + chemotherapy(18) and chemotherapy(16) 4 LLN = lower limit of normal Biliary Tract Cancer Locally advanced or metastatic BTC -TOPAZ-1 The safety of IMFINZI in combination with gemcitabine and cisplatin in locally advanced or metastatic BTC was evaluated in TOPAZ-1, a randomized, double-blind, placebo-controlled, multicenter trial. A total of 338 patients received IMFINZI 1,500 mg in combination with gemcitabine and cisplatin every 3 weeks up to 8 cycles followed by IMFINZI 1,500 mg every 4 weeks until disease progression or unacceptable toxicity. Patients with active or prior documented autoimmune or inflammatory disorders, HIV infection or other active infections, including tuberculosis or hepatitis C were ineligible [see Clinical Studies (14.4)]. IMFINZI was discontinued due to adverse reactions in 6% of the patients receiving IMFINZI plus chemotherapy. The most frequently reported events resulting in discontinuation were sepsis (3 patients) and ischemic stroke (2 patients). The remaining events were dispersed across system organ classes and reported in 1 patient each. Serious adverse reactions occurred in 47% of patients receiving IMFINZI plus chemotherapy. The most frequent serious adverse reactions reported in at least 2% of patients were cholangitis (7%), pyrexia (3.8%), anemia (3.6%), sepsis (3.3%) and acute kidney injury (2.4%). Fatal adverse reactions occurred in 3.6% of patients receiving IMFINZI plus chemotherapy. These include ischemic or hemorrhagic stroke (4 patients), sepsis (2 patients), upper gastrointestinal hemorrhage (2 patients). The most common adverse reactions (occurring in ≥ 20% of patients) were fatigue, nausea, constipation, decreased appetite, abdominal pain, rash and pyrexia. Table 13 summarizes the adverse reactions that occurred in patients treated with IMFINZI plus chemotherapy. Table 13. Adverse Reactions Occurring in ≥ 10% of Patients in the TOPAZ-1 Study IMFINZI with Gemcitabine Placebo with Gemcitabine and Cisplatin and Cisplatin N = 338 N = 342 Adverse Reaction All Grades* Grade* 3-4 (%) All Grades* Grade* 3-4 (%) (%) (%) General disorders and administration site conditions Fatigue† 42 6 43 6 Pyrexia 20 1.5 16 0.6 Gastrointestinal disorders Nausea 40 1.5 34 1.8 Constipation 32 0.6 29 0.3 Abdominal pain‡ 24 0.6 23 2.9 Vomiting 18 1.5 18 2.0 Diarrhea 17 1.2 15 1.8 Metabolism and nutrition disorders Decreased appetite 26 2.1 23 0.9 Skin and subcutaneous tissue disorders Rash§ 23 0.9 14 0 Pruritus 11 0 8 0 Psychiatric disorders Insomnia 10 0 11 0 * Graded according to NCI CTCAE version 5.0 † Includes fatigue, malaise, cancer fatigue and asthenia. ‡ Includes abdominal pain, abdominal pain lower, abdominal pain upper and flank pain. § Includes rash macular, rash maculopapular, rash morbilliform, rash papular, rash pruritic, rash pustular, rash erythematous, dermatitis acneiform, dermatitis bullous, drug eruption, eczema, erythema, dermatitis and rash. Table 14 summarizes the laboratory abnormalities in patients treated with IMFINZI plus chemotherapy. Table 14. Laboratory Abnormalities Worsening from Baseline Occurring in ≥ 20%* of Patients in the TOPAZ-1 study IMFINZI with Gemcitabine and Placebo with Cisplatin Gemcitabine and Cisplatin Laboratory Abnormality Grade† 3 or 4 Grade† 3 or 4 (%) (%) Chemistry Hyponatremia 18 13 Gamma-glutamyltransferase 12 13 increased Increased bilirubin 10 14 Hypokalemia 8 4.4 Increased AST 8 8 Increased ALT 7 6 Blood creatinine increased 5 2.1 Hypomagnesemia 4.5 2.2 Hypoalbuminemia 3.6 2.9 Hyperkalemia 2.1 2.1 Increased Alkaline Phosphatase 1.8 3.8 Hypocalcemia 1.8 2.4 Hematology Neutropenia 48 49 Anemia 31 28 Leukopenia 28 28 Lymphopenia 23 15 Thrombocytopenia 18 18 * The frequency cut off is based on any grade change from baseline † Graded according to NCI CTCAE version 5.0. Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: IMFINZI + Gem/Cis (range: 312 to 335) and Placebo + Gem/Cis (range: 319 to 341). Hepatocellular Carcinoma Unresectable HCC - HIMALAYA The safety of IMFINZI in combination with tremelimumab was evaluated in a total of 388 patients with uHCC in HIMALAYA, a randomized, open-label, multicenter study [see Clinical Studies (14.1)]. Patients received IMFINZI 1,500 mg administered as a single intravenous infusion in combination with tremelimumab 300 mg on the same day, followed by IMFINZI every 4 weeks or sorafenib 400 mg given orally twice daily. Serious adverse reactions occurred in 41% of patients who received IMFINZI in combination with tremelimumab. Serious adverse reactions in > 1% of patients included hemorrhage (6%), diarrhea (4%), sepsis (2.1%), pneumonia (2.1%), rash (1.5%), vomiting (1.3%), acute kidney injury (1.3%), and anemia (1.3%). Fatal adverse reactions occurred in 8% of patients who received IMFINZI in combination with tremelimumab, including death (1%), hemorrhage intracranial (0.5%), cardiac arrest (0.5%), pneumonitis (0.5%), hepatic failure (0.5%), and immune-mediated hepatitis (0.5%). The most common adverse reactions (occurring in ≥ 20% of patients) were rash, diarrhea, fatigue, pruritis, musculoskeletal pain, and abdominal pain. Permanent discontinuation of treatment regimen due to an adverse reaction occurred in 14% of patients; the most common adverse reactions leading to treatment discontinuation (≥ 1%) were hemorrhage (1.8%), diarrhea (1.5%), AST increased (1%), and hepatitis (1%). Dosage interruptions or delay of the treatment regimen due to an adverse reaction occurred in 35% of patients. Adverse reactions which required dosage interruption or delay in ≥ 1% of patients included ALT increased (3.6%), diarrhea (3.6%), rash (3.6%), amylase increased (3.4%), AST increased (3.1%), lipase increased (2.8%), pneumonia (1.5%), hepatitis (1.5%), pyrexia (1.5%), anemia (1.3%), thrombocytopenia (1%), hyperthyroidism (1%), pneumonitis (1%), and blood creatinine increased (1%). Table 15 summarizes the adverse reactions that occurred in patients treated with IMFINZI in combination with tremelimumab in the HIMALAYA study. Table 15. Adverse Reactions Occurring in ≥ 10% of Patients in the HIMALAYA study IMFINZI and Sorafeni Tremelimumab b (N=388) (N=374) All Grade 3- All Grades Grade 3- Adverse Reaction (%) Grade 4 (%) 4 (%) s (%) Gastrointestinal disorders Diarrhea* 27 6 45 4.3 Abdominal pain* 20 1.8 24 4 Nausea 12 0 14 0 Skin and subcutaneous tissue disorders Rash* 32 2.8 57 12 Pruritus 23 0 6 0.3 Metabolism and nutrition disorders Decreased appetite 17 1.3 18 0.8 General disorders and administration site conditions Fatigue* 26 3.9 30 6 Pyrexia* 13 0.3 9 0.3 Psychiatric disorders Insomnia 10 0.3 4.3 0 Endocrine disorders Hypothyroidism* 14 0 6 0 Musculoskeletal and Connective Tissue Disorders Musculoskeletal pain* 22 2.6 17 0.8 * Represents a composite of multiple related terms. Table 16 summarizes the laboratory abnormalities that occurred in patients treated with IMFINZI in combination with tremelimumab in the HIMALAYA study. Table 16. Laboratory Abnormalities Worsening from Baseline Occurring in ≥ 20% of Patients in the HIMALAYA study IMFINZI and Sorafeni Tremelimumab b Laboratory Abnormality Any Grade 3† or 4 Any Grade 3† or 4 grade† (%)‡ grade† (%)‡ (%)‡ (%)‡ Chemistry Aspartate 63 27 55 21 Aminotransferase increased Alanine Aminotransferase 56 18 53 12 increased Sodium decreased 46 15 40 11 Bilirubin increased 41 8 47 11 Alkaline 8 44 5 41 Phosphatase increased Glucose increased 39 14 29 4 Calcium decreased 34 0 43 0.3 Albumin decreased 31 0.5 37 1.7 Potassium increased 28 3.8 21 2.6 Creatinine increased 21 1.3 15 0.9 Hematology Hemoglobin decreased 52 4.8 40 6 Lymphocytes decreased 41 11 39 10 Platelets decreased 29 1.6 35 3.1 Leukocytes decreased 20 0.8 30 1.1 † Graded according to NCI CTCAE version 4.03. ‡ Each test incidence is based on the number of patients who had both baseline and at least one on- study laboratory measurement available: IMFINZI with tremelimumab (range: 367-378) and sorafenib (range:344-352). Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorization of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form: https://sideeffects.health.gov.il 8. USE IN SPECIFIC POPULATIONS 8.1 Pregnancy Risk summary Based on findings from animal studies and its mechanism of action, IMFINZI can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data on the use of IMFINZI in pregnant women. In animal reproduction studies, administration of durvalumab to pregnant cynomolgus monkeys from the confirmation of pregnancy through delivery at exposure levels approximately 6 to 20 times higher than those observed at the clinical dose of 10 mg/kg based on area under the curve (AUC), resulted in an increase in premature delivery, fetal loss and premature neonatal death (see Data). Human immunoglobulin G1 (IgG1) is known to cross the placental barrier; therefore, durvalumab has the potential to be transmitted from the mother to the developing fetus. Apprise pregnant women of the potential risk to a fetus. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively. Data Animal Data As reported in the literature, the PD-1/PD-L1 pathway plays a central role in preserving pregnancy by maintaining maternal immune tolerance to the fetus. In mouse allogeneic pregnancy models, disruption of PD-L1 signaling was shown to result in an increase in fetal loss. The effects of durvalumab on prenatal and postnatal development were evaluated in reproduction studies in cynomolgus monkeys. Durvalumab was administered from the confirmation of pregnancy through delivery at exposure levels approximately 6 to 20 times higher than those observed at a clinical dose of 10 mg/kg (based on AUC). Administration of durvalumab resulted in premature delivery, fetal loss (abortion and stillbirth) and increase in neonatal deaths. Durvalumab was detected in infant serum on postpartum Day 1, indicating the presence of placental transfer of durvalumab. Based on its mechanism of action, fetal exposure to durvalumab may increase the risk of developing immune-mediated disorders or altering the normal immune response and immune-mediated disorders have been reported in PD-1 knockout mice. 8.2 Lactation Risk Summary There are no data on the presence of durvalumab in human milk, its effects on the breastfed child, or the effects on milk production. Maternal IgG is known to be present in human milk. The effects of local gastrointestinal exposure and limited systemic exposure in the breastfed child to IMFINZI are unknown. Durvalumab was present in the milk of lactating cynomolgus monkeys and was associated with premature neonatal death (see Data). Because of the potential for adverse reactions in a breastfed child, advise women not to breastfeed during treatment with IMFINZI and for 3 months after the last dose. Refer to the Prescribing Information for the agents administered in combination with IMFINZI for recommended duration to not breastfeed, as appropriate. Data In lactating cynomolgus monkeys, durvalumab was present in breast milk at about 0.15% of maternal serum concentrations after administration of durvalumab from the confirmation of pregnancy through delivery at exposure levels approximately 6 to 20 times higher than those observed at the recommended clinical dose of 10 mg/kg (based on AUC). Administration of durvalumab resulted in premature neonatal death. 8.3 Females and Males of Reproductive Potential Pregnancy testing Verify pregnancy status of females of reproductive potential prior to initiating treatment with IMFINZI. Contraception Females IMFINZI can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with IMFINZI, and for 3 months following the last dose of IMFINZI. Refer to the Prescribing Information for the agents administered in combination with IMFINZI for recommended contraception duration, as appropriate. 8.4 Pediatric Use The safety and effectiveness of IMFINZI have not been established in pediatric patients. 8.5 Geriatric Use No dose adjustment is required for elderly patients (≥ 65 years of age) (see section 14). Of the 476 patients treated with IMFINZI in the PACIFIC study, 45% were 65 years or older, while 7.6% were 75 years or older. No overall differences in safety or effectiveness were observed between patients 65 years or older and younger patients. The PACIFIC study did not include sufficient numbers of patients aged 75 years and over to determine whether they respond differently from younger patients. Of the 265 patients with ES-SCLC treated with IMFINZI in combination with chemotherapy 101 (38%) patients were 65 years or older and 19 (7.2%) patients were 75 years or older. There were no clinically meaningful differences in safety or efficacy between patients 65 years or older and younger patients. Of the 330 patients with metastatic NSCLC treated with IMFINZI in combination with tremelimumab and platinum-based chemotherapy, 143 (43%) patients were 65 years or older and 35 (11%) patients were 75 years or older. There were no clinically meaningful differences in safety or efficacy between patients 65 years or older and younger patients. Of the 338 patients with BTC treated with IMFINZI in combination with chemotherapy in the TOPAZ-1 study, 158 (47%) patients were 65 years or older and 38 (11%) patients were 75 years or older. No overall differences in safety or effectiveness of IMFINZI have been observed between patients 65 years of age and older and younger adult patients. Of the 393 patients with uHCC treated with IMFINZI in combination with tremelimumab, 50% of patients were 65 years of age or older and 13% of patients were 75 years of age or older. No overall differences in safety or effectiveness of IMFINZI have been observed between patients 65 years of age and older and younger adult patients. In studies PACIFIC, CASPIAN and TOPAZ-1 data on safety for patients 75 years and older are too limited to draw a conclusion on this population. In first line metastatic NSCLC patients in the POSEIDON study, some differences in safety were reported between elderly (≥ 65 years) and younger patients. The safety data from patients 75 years of age or older are limited to a total of 74 patients. There was a higher frequency of serious adverse reactions and discontinuation rate of any study treatment due to adverse reactions in 35 patients aged 75 years of age or older treated with IMFINZI in combination with tremelimumab and platinum-based chemotherapy (45.7% and 28.6%, respectively) relative to 39 patients aged 75 years of age or older who received platinum-based chemotherapy only (35.9% and 20.5%, respectively).

פרטי מסגרת הכללה בסל
א. התרופה תינתן לטיפול במקרים האלה:1. כמונותרפיה לטיפול בסרטן מתקדם מקומי או גרורתי של דרכי השתן בחולה עם PDL1 גבוה (TC > 25%) והעונה על אחד מאלה: א. מחלתו התקדמה לאחר שקיבל טיפול כימותרפי קודם במשטר שכלל תרכובת פלטינום למחלתו הגרורתית;ב. מחלתו התקדמה בתוך 12 חודשים מטיפול כימותרפי במשטר שכלל תרכובת פלטינום במסגרת משלימה (adjuvant) או noeoadjuvant. במהלך מחלתו יהיה החולה זכאי לתרופה אחת בלבד מתרופות המשתייכות למשפחת ה-Checkpoint inhibitors2. כמונותרפיה בסרטן ריאה מסוג NSCLC שלב III לא נתיח, בחולים שמחלתם לא התקדמה לאחר טיפול משולב בכימותרפיה מבוססת פלטינום והקרנות.משך הטיפול בתכשיר להתוויה זו לא יעלה על שנה.במהלך מחלתו יהיה החולה זכאי לתרופה אחת בלבד מתרופות המשתייכות למשפחת ה-Checkpoint inhibitors.לעניין זה סרטן ריאה מסוג NSCLC שלב III לא נתיח לא מוגדר כאותה מחלה כמו סרטן ריאה מסוג NSCLC בשלב IV.3. בשילוב עם Tremelimumab במחזור הראשון לטיפול, לטיפול בסרטן הפטוצלולארי לא נתיח או גרורתי, בחולים שטרם קיבלו טיפול סיסטמי למחלתם.במהלך מחלתו יהיה החולה זכאי לאחת מהתרופות הבאות – Atezolizumab + Bevacizumab, Lenvatinib, Sorafenib, Durvalumab + Tremelimumab. במהלך מחלתו יהיה החולה זכאי לתרופה אחת בלבד מתרופות המשתייכות למשפחת ה-Checkpoint inhibitors (לעניין זה שילוב Durvalumab עם Tremelimumab יחשב תרופה אחת).ב. מתן התרופה האמורה ייעשה לפי מרשם של רופא מומחה באונקולוגיה או רופא מומחה באורולוגיה המטפל באורולוגיה אונקולוגית.
מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| סרטן ריאה מסוג NSCLC שלב III לא נתיח, בחולים שמחלתם לא התקדמה לאחר טיפול משולב בכימותרפיה והקרנות. משך הטיפול בתכשיר להתוויה זו לא יעלה על שנה. במהלך מחלתו יהיה החולה זכאי לתרופה אחת בלבד מתרופות המשתייכות למשפחת ה-Checkpoint inhibitors. לעניין זה סרטן ריאה מסוג NSCLC שלב III לא נתיח לא מוגדר כאותה מחלה כמו סרטן ריאה מסוג NSCLC בשלב IV. | 16/01/2019 | אונקולוגיה | סרטן ריאה מתקדם, Non small cell lung cancer | |
| התרופה תינתן כמונותרפיה לטיפול בסרטן מתקדם מקומי או גרורתי של דרכי השתן בחולה עם PDL1 גבוה (TC > 25%) והעונה על אחד מאלה: 1. קיבל טיפול כימותרפי קודם במשטר שכלל תרכובת פלטינום למחלתו הגרורתית; 2. מחלתו התקדמה בתוך 12 חודשים מטיפול כימותרפי במשטר שכלל תרכובת פלטינום במסגרת משלימה (adjuvant) או noeoadjuvant. במהלך מחלתו יהיה החולה זכאי לתרופה אחת בלבד מתרופות המשתייכות למשפחת ה-Checkpoint inhibitors | 11/01/2018 | אונקולוגיה | Urothelial cancer, סרטן מתקדם בדרכי השתן | |
| מונותרפיה לטיפול בסרטן מתקדם מקומי או גרורתי של דרכי השתן בחולה עם PDL1 גבוה (TC > 25%) והעונה על אחד מאלה: א. מחלתו התקדמה לאחר שקיבל טיפול כימותרפי קודם במשטר שכלל תרכובת פלטינום למחלתו הגרורתית; ב. מחלתו התקדמה בתוך 12 חודשים מטיפול כימותרפי במשטר שכלל תרכובת פלטינום במסגרת משלימה (adjuvant) או noeoadjuvant. במהלך מחלתו יהיה החולה זכאי לתרופה אחת בלבד מתרופות המשתייכות למשפחת ה-Checkpoint inhibitors | 03/02/2022 | אונקולוגיה | Urothelial cancer, סרטן מתקדם בדרכי השתן | |
| א. התרופה תינתן לטיפול במקרים האלה: 1. כמונותרפיה לטיפול בסרטן מתקדם מקומי או גרורתי של דרכי השתן בחולה עם PDL1 גבוה (TC > 25%) והעונה על אחד מאלה: א. מחלתו התקדמה לאחר שקיבל טיפול כימותרפי קודם במשטר שכלל תרכובת פלטינום למחלתו הגרורתית; ב. מחלתו התקדמה בתוך 12 חודשים מטיפול כימותרפי במשטר שכלל תרכובת פלטינום במסגרת משלימה (adjuvant) או noeoadjuvant. במהלך מחלתו יהיה החולה זכאי לתרופה אחת בלבד מתרופות המשתייכות למשפחת ה-Checkpoint inhibitors 2. כמונותרפיה בסרטן ריאה מסוג NSCLC שלב III לא נתיח, בחולים שמחלתם לא התקדמה לאחר טיפול משולב בכימותרפיה מבוססת פלטינום והקרנות. משך הטיפול בתכשיר להתוויה זו לא יעלה על שנה. במהלך מחלתו יהיה החולה זכאי לתרופה אחת בלבד מתרופות המשתייכות למשפחת ה-Checkpoint inhibitors. לעניין זה סרטן ריאה מסוג NSCLC שלב III לא נתיח לא מוגדר כאותה מחלה כמו סרטן ריאה מסוג NSCLC בשלב IV. 3. בשילוב עם Tremelimumab במחזור הראשון לטיפול, לטיפול בסרטן הפטוצלולארי לא נתיח או גרורתי, בחולים שטרם קיבלו טיפול סיסטמי למחלתם. במהלך מחלתו יהיה החולה זכאי לאחת מהתרופות הבאות – Atezolizumab + Bevacizumab, Lenvatinib, Sorafenib, Durvalumab + Tremelimumab. במהלך מחלתו יהיה החולה זכאי לתרופה אחת בלבד מתרופות המשתייכות למשפחת ה-Checkpoint inhibitors (לעניין זה שילוב Durvalumab עם Tremelimumab יחשב תרופה אחת). | 17/03/2024 | אונקולוגיה | סרטן מתקדם מקומי או גרורתי של דרכי השתן |
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
11/01/2018
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
