Quest for the right Drug
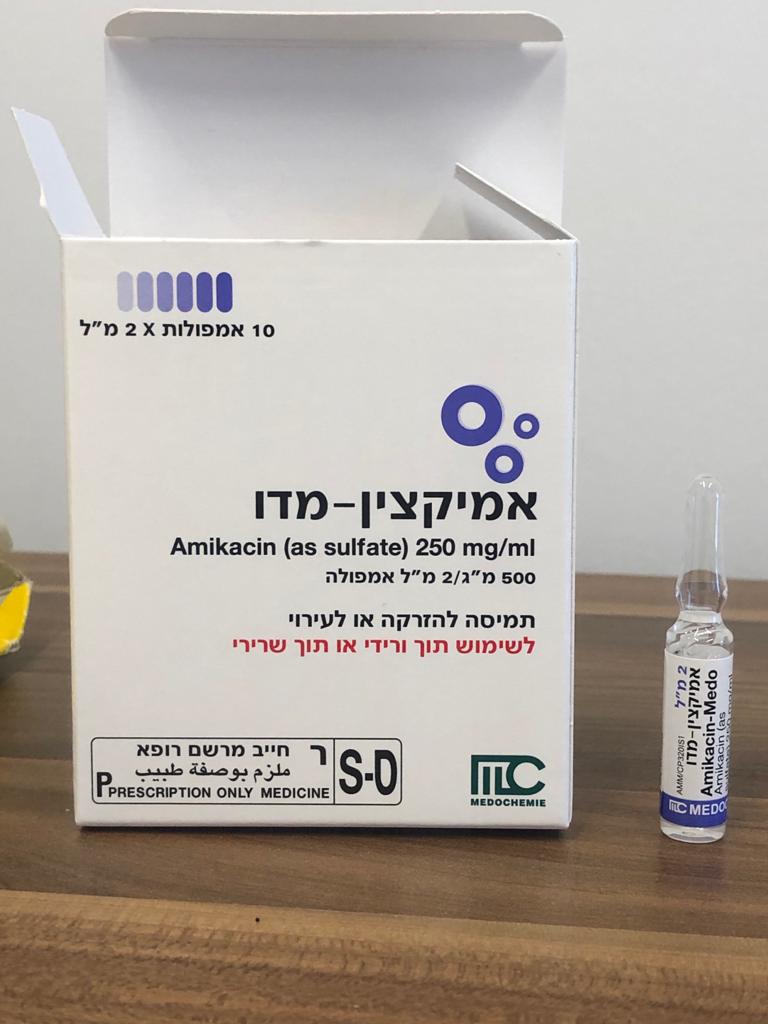
אמיקצין-מדו AMIKACIN - MEDO (AMIKACIN AS SULFATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-שרירי, תוך-ורידי : I.M, I.V
צורת מינון:
תמיסה להזרקהאינפוזיה : SOLUTION FOR INJECTION / INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use Patients should be well hydrated during amikacin therapy. Caution should be applied to patients with pre-existing renal insufficiency, pre-existing hearing or vestibular damage and diminished glomerular filtration. Patients treated with parenteral aminoglycosides should be under close clinical observation because of the potential ototoxicity and nephrotoxicity associated with their use. Safety for treatment periods which are longer than 14 days has not been established. If therapy is expected to last seven days or more in patients with renal impairment, or 10 days in other patients, a pre-treatment audiogram should be obtained and repeated during therapy. Renal Toxicity Aminoglycosides are potentially nephrotoxic. Renal toxicity is independent of plasma obtained at the peak (Cmax). The risk of nephrotoxicity is greater in patients with impaired renal function, and in those who receive higher doses, or in those whose therapy is prolonged. Patients should be well hydrated during treatment and renal function should be assessed by the usual methods prior to starting therapy and daily during the course of treatment. A reduction of dosage is required if evidence of renal dysfunction occurs, such as presence of urinary casts, white or red cells, albuminuria, decreased creatinine clearance, decreased urine specific gravity, increased BUN, serum creatinine, or oliguria. If azotemia increases, or if a progressive decrease in urinary output occurs, treatment should be stopped. Elderly patients may have reduced renal function which may not be evident in routine screening tests such as BUN or serum creatinine. A creatinine clearance determination may be more useful. Monitoring of renal function in elderly patients during treatment with aminoglycosides is particularly important. Renal and eighth-cranial nerve function should be closely monitored especially in patients with known or suspected renal impairment at the onset of therapy, and also in those whose renal function is initially normal but who develop signs of renal dysfunction during therapy. Serum concentrations of amikacin should be monitored when feasible to assure adequate levels and to avoid potentially toxic levels. Urine should be examined for decreased specific gravity, increased excretion of proteins, and the presence of cells or casts. Blood urea nitrogen, serum creatinine, or creatinine clearance should be measured periodically. Serial audiograms should be obtained where feasible in patients old enough to be tested, particularly high risk patients. Evidence of ototoxicity (dizziness, vertigo, tinnitus, roaring in the ears, and hearing loss) or nephrotoxicity requires discontinuation of the drug or dosage adjustment. Concurrent and/or sequential, oral, or topical use of other neurotoxic or nephrotoxic products, particularly bacitracin, cisplatin, amphotericin B, cephaloridine, paromomycin, viomycin, polymyxin B, colistin, vancomycin, or other aminoglycosides, should be avoided. Other factors that may increase risk of toxicity are advanced age and dehydration. Patients suffering from pre-existing renal insufficiency should be assessed by the usual methods prior to therapy and periodically during therapy. Daily doses should be reduced and/or the interval between doses lengthened in accordance with serum creatinine concentrations to avoid accumulation of abnormally high blood levels and to minimise the risk of ototoxicity. Regular monitoring of serum drug concentration and of renal function is particularly important in elderly patients, who may have reduced renal function that may not be evident in the results of routine screening tests i.e. blood urea and serum creatinine. Neurotoxicity Neurotoxicity, manifested as vestibular and/or bilateral ototoxicity, can occur in patients treated with aminoglycosides. The risk of aminoglycoside-induced ototoxicity is greater in patients with impaired renal function, and in those who receive high doses, or in those whose therapy is prolonged over 5-7 days of treatment, even in healthy patients. High frequency deafness usually occurs first and can be detected only by audiometric testing. Vertigo may occur and may be evidence of vestibular injury. Other manifestations of neurotoxicity may include numbness, skin tingling, muscle twitching and convulsions. Ototoxicity The risk of ototoxicity due to aminoglycosides increases with the degree of exposure to either persistently high peak or high trough serum concentrations. Patients developing cochlear or vestibular damage may not have symptoms during therapy to warn them of developing eighth nerve toxicity, and total or partial irreversible bilateral deafness or disabling vertigo may occur after the drug has been discontinued. Aminoglycoside-induced ototoxicity is usually irreversible. Patients with mitochondrial DNA mutations, particularly the nucleotide 1555 A to G substitution in the 12S rRNA gene may be at higher risk for ototoxicity, even if the patient's aminoglycoside serum levels were within the recommended range. In case of family history of aminoglycoside-induced deafness or known mitochondrial DNA mutations in the 12S rRNA gene, alternative treatments other than aminoglycosides should be considered. Neuromuscular Toxicity Neuromuscular blockade and respiratory paralysis have been reported following parenteral injection, topical instillation (as in orthopaedic and abdominal irrigation or in local treatment of empyema), and following oral use of aminoglycosides. The possibility of respiratory paralysis should be considered if aminoglycosides are administered by any route, especially in patients receiving anaesthetics, neuromuscular blocking agents such as tubocurarine, succinylcholine, decamethonium, atracurium, rocuronium, vecuronium or in patients receiving massive transfusions of citrate-anticoagulated blood. If neuromuscular blockade occurs, calcium salts may reverse respiratory paralysis, but mechanical respiratory assistance may be necessary. Neuromuscular blockade and muscular paralysis have been demonstrated in laboratory animals given high doses of amikacin. Amikacin must not be used in patients with myasthenia gravis. Aminoglycosides should be used with caution in patients with muscular disorders such as Parkinsonism since these drugs may aggravate muscle weakness because of their potential curare-like effect on the neuromuscular junction. Allergic reactions The use of amikacin in patients with a history of allergy to aminoglycosides or in patients who may have subclinical renal or eighth nerve damage induced by prior administration of nephrotoxic and/or ototoxic agents such as streptomycin, dihydrostreptomycin, gentamicin, tobramycin, kanamycin, neomycin, polymyxin B, colistin, cephaloridine or viomycin should be considered with caution, as toxicity may be additive. In these patients amikacin should be used only if, in the opinion of the physician, therapeutic advantages outweigh the potential risks. Large doses of amikacin administered during surgery have been responsible for a transient myasthenic syndrome. Amikacin sulfate injection in ampoules contains sodium metabisulphite, a sulphite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulphite sensitivity in the general population is uncommon and probably low. Sulphite sensitivity is seen more frequently in asthmatic than in non-asthmatic subjects. Paediatric use Aminoglycosides should be used with caution in premature and neonatal infants because of the renal immaturity of these patients and the resulting prolongation of serum half-life of these drugs. Other Aminoglycosides are quickly and almost totally absorbed when they are applied topically, except to the urinary bladder, in association with surgical procedures. Irreversible deafness, renal failure and death due to neuromuscular blockade have been reported following irrigation of both small and large surgical fields with an aminoglycoside preparation. As with other antibiotics, the use of amikacin may result in overgrowth of non-susceptible organisms. If this occurs, appropriate therapy should be instituted. Amikacin-Medo 500mg/2ml solution for injection/infusion contains 0.65 mmol sodium per 2 ml. This medicinal product contains less than 1 mmol sodium (23 mg) per ml, i.e. essentially ‘sodium-free’.
Effects on Driving
4.7 Effects on ability to drive and use machines No studies on the effects on the ability to drive and use machines have been performed. Due to the occurrence of some adverse reactions (see section 4.8) the ability to drive and use machines may be impaired.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
