Quest for the right Drug
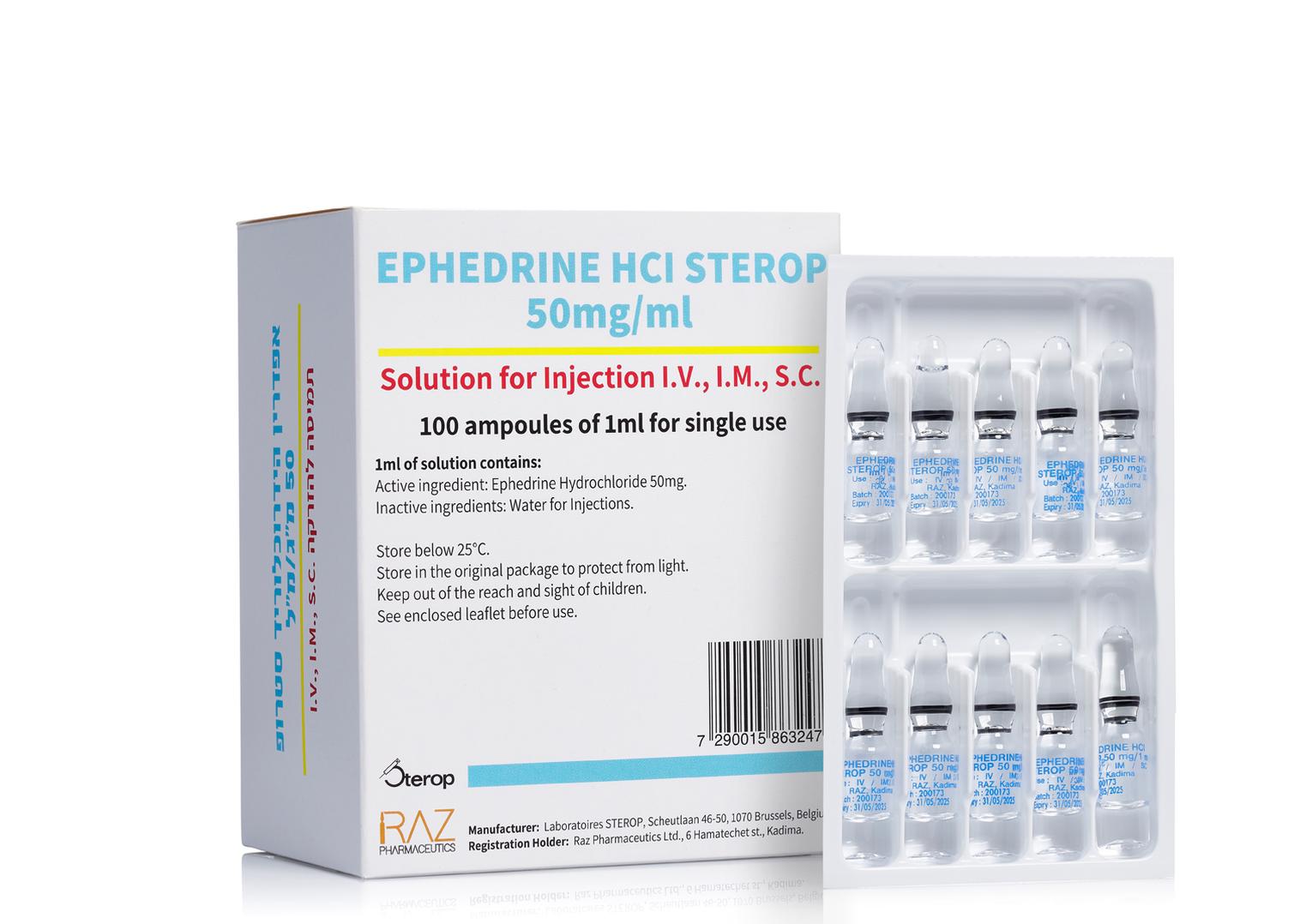
אפדרין הידרוכלוריד סטרופ 50מ"ג/1מ"ל EPHEDRINE HCL STEROP 50 MG/1 ML (EPHEDRINE HYDROCHLORIDE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי, תוך-שרירי, תוך-ורידי : S.C, I.M, I.V
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmaceutical particulars : מידע רוקחי
6. PHARMACEUTICAL PARTICULARS 6.1 List of excipients Water for injections. 6.2 Incompatibilities EPHEDRINE HCl STEROP 50mg/1ml must not be mixed with other medicinal products except those mentioned in section 6.6. 6.3 Shelf life Unopened: The expiry date of the product is indicated on the packaging material. After first opening: the product must be used immediately. After dilution: For storage conditions and shelf life after dilution see section 6.6. 6.4 Special precautions for storage Do not store above 25˚C. Store in the original package in order to protect from light. 6.5 Nature and contents of container Type I uncolored glass flat bottom ampoule. Boxes of 5, 10 or 100 ampoules containing 1ml of solution. 6.6 Special precautions for disposal and other handling Instructions for use: The ampoule is for single use only. The solution should be used immediately after the opening of the container. The drug product EPHEDRINE HCl STEROP 50 mg/1 ml solution for injection is stable for 24 hours when diluted with sodium chloride 0.9 % solution for injection at room temperature (15 -25℃), without protection from light, when diluted as follows: 1. One ampoule is diluted to a final volume of 5 ml (4 ml of saline + 1 ml of ephedrine). 2. One ampoule is diluted to a final volume of 10 ml (9 ml of saline + 1 ml of ephedrine). Chemical and physical in-use stability has been demonstrated for 24 hours at room temperature (15 - 25ºC). From a microbiological point of view, unless the method of opening/dilution precludes the risk of microbial contamination, the diluted product should be used immediately after preparation. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user. Any unused product or waste material should be disposed of in accordance with local requirements. Discard the ampoule after use. DO NOT REUSE. The content of un-opened and un-damaged ampoule is sterile and must not be opened until use. The product should be inspected visually for particles and discoloration prior to administration. Only clear colorless solution free from particles or precipitates should be used.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
עלון מידע לצרכן
02.02.24 - החמרה לעלוןלתרופה במאגר משרד הבריאות
אפדרין הידרוכלוריד סטרופ 50מ"ג/1מ"ל