Quest for the right Drug
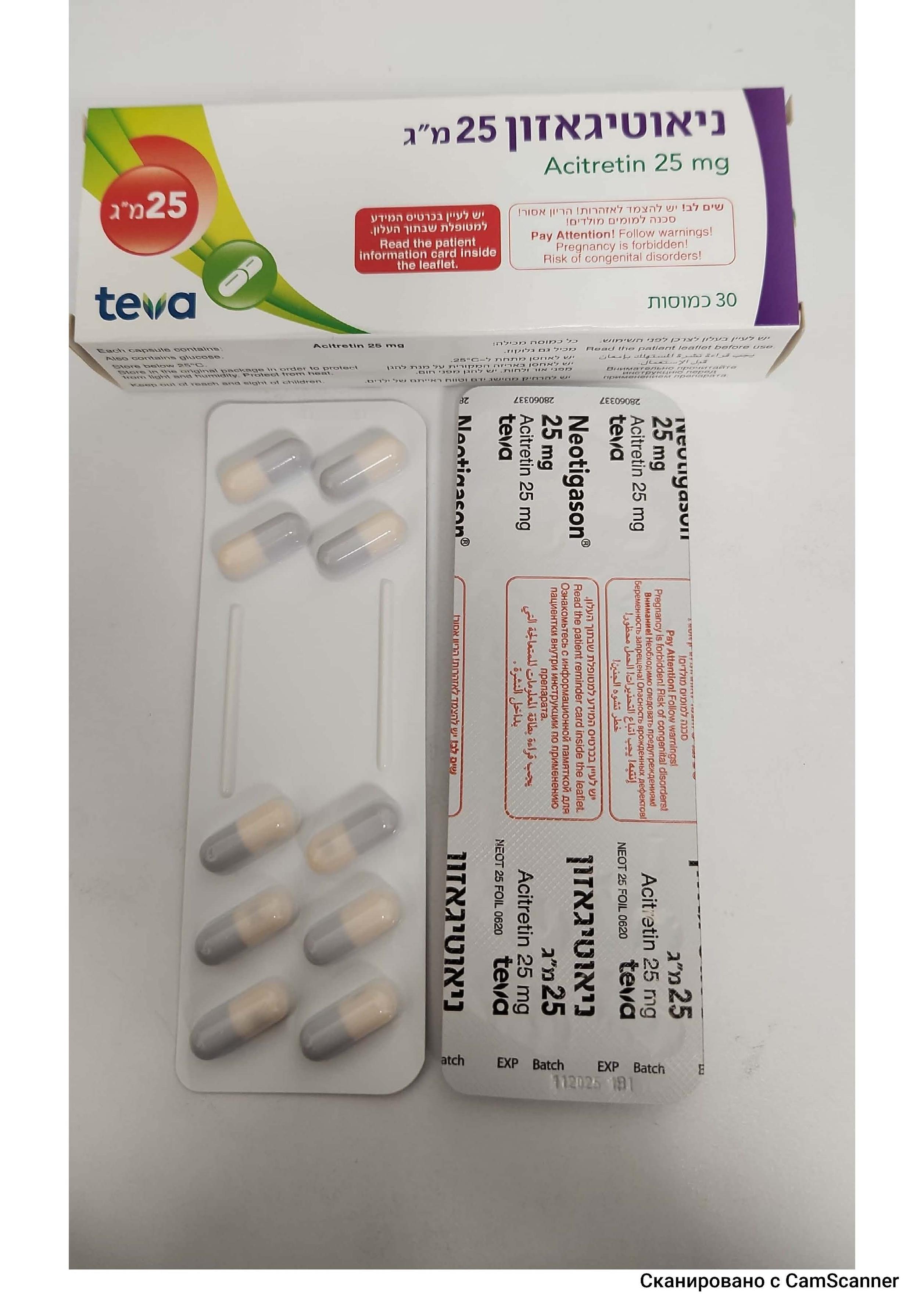
ניאוטיגאזון 25 מ"ג NEOTIGASON 25 MG (ACITRETIN)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
קפסולות : CAPSULES
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pregnancy & Lactation : הריון/הנקה
4.6 Fertility, Pregnancy and lactation Women of childbearing potential / Contraception in males and females Neotigason is highly teratogenic. Its use is contraindicated in women who might become pregnant during or within 3 years of the cessation of treatment. The risk of giving birth to a deformed child is exceptionally high if acitretin is taken before or during pregnancy, no matter for how long or at what dosage. Neotigason is contraindicated in every woman of childbearing potential unless each of the following conditions is met: 1) The patient is suffering from a severe disorder of keratinisation which is resistant to standard therapies. 2) She can be relied on to understand and follow the physician’s instructions. Neotigason 10mg, 25mg MF 11/2024 Notification CLEAN 3) She is capable of taking the stipulated contraceptive measures reliably and without fail. 4) It is absolutely essential that every woman of childbearing potential who is to undergo treatment with acitretin uses effective contraception (preferably 2 complementary methods) without interruption for four weeks before, during and for 3 years after the discontinuation of treatment with acitretin. The patient should be instructed to immediately contact a doctor in case of suspected pregnancy. Even female patients who normally do not practice contraception because of a history of infertility should be advised to do so, while taking Neotigason. 5) Therapy should not begin until the second or third day of the next normal menstrual period. 6) At the start of therapy, a negative pregnancy test result (minimum sensitivity of 25mIU/mL) must be obtained up to three days before the first dose is given. During therapy, pregnancy tests should be arranged at 28-day intervals. A negative pregnancy test not older than 3 days is mandatory before prescription is made at these visits. After stopping therapy, pregnancy tests should be performed at 1-3 monthly intervals for a period of 3 years after the last dose is given. 7) Before therapy with acitretin is instituted, the physician must give patients of childbearing potential detailed information about the precautions to be taken, the risk of very severe foetal malformation, and the possible consequences if pregnancy occurs during the course of treatment with acitretin or within 3 years of discontinuing therapy. 8) The same effective and uninterrupted contraceptive measures must be taken every time therapy is repeated, however long the intervening period may have been, and must be continued for 3 years afterwards. 9) Should pregnancy occur, in spite of these precautions, there is a high risk of severe malformation of the foetus (e.g. craniofacial defects, cardiac and vascular or CNS malformations, skeletal and thymic defects) and the incidence of spontaneous abortion is increased. This risk applies especially during treatment with acitretin and 2 months after treatment. For up to 3 years after acitretin discontinuation, the risk is lower (particularly in women who have not consumed alcohol) but cannot be entirely excluded (due to possible formation of etretinate). Therefore, before instituting Neotigason the treating physician must explain clearly and in detail what precautions must be taken. This should include the risks involved and the possible consequences of pregnancy occurring during Neotigason treatment or in the 3 years following its cessation. 10) Women of childbearing age must not consume alcohol (in drinks, food or medicines) during treatment with acitretin and for 2 months after cessation of acitretin therapy (see section 4.4, 4.5 and 5.2). Neotigason 10mg, 25mg MF 11/2024 Notification CLEAN Primary contraceptive method is a combination hormonal contraceptive product or an intrauterine device and it is recommended that a condom or diaphragm (cap) is also used. Low dose progesterone-only products (minipills) are not recommended due to indications of possible interference with their contraceptive effect. For male patients treated with acitretin, available data, based on the level of maternal exposure from the semen and seminal fluid indicate a minimal, if any, risk of teratogenic effects. Pregnancy Neotigason is contraindicated in pregnant women (see section 4.3). Breastfeeding Neotigason must not be given to nursing mothers (see section 4.3).

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/03/2002
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
