Quest for the right Drug
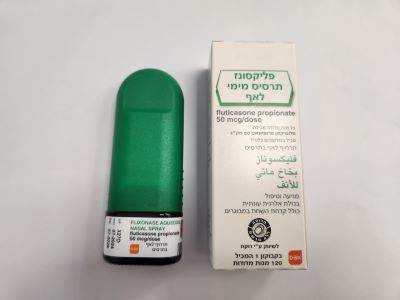
פליקסונז תרסיס מימי לאף FLIXONASE AQUEOUS NASAL SPRAY (FLUTICASONE PROPIONATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
אפי : NASAL
צורת מינון:
אין פרטים : NASAL SPRAY, SUSPENSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects Adverse events are listed below by system organ class and frequency. Frequencies are defined as: very common (≥1/10), common (≥1/100 and <1/10), uncommon (≥1/1000 and <1/100), rare (≥1/10,000 and <1/1000) and very rare (<1/10,000) including isolated reports and not known (cannot be estimated from the available data). Very common, common and uncommon events were generally determined from clinical trial data. Rare and very rare events were generally determined from spontaneous data. In assigning adverse event frequencies, the background rates in placebo groups were not taken into account. System Organ Class Adverse Event Frequency Immune system Hypersensitivity reactions with the Very rare disorders following manifestations: Cutaneous hypersensitivity reactions Very rare Angioedema (mainly facial and Very rare oropharyngeal oedema) Respiratory symptoms (bronchospasm) Very rare Anaphylactic reactions Very rare Nervous system, Headache, unpleasant taste, unpleasant Common disorders smell Eye disorders Glaucoma, raised intraocular pressure, Very rare cataract These events have been identified from spontaneous reports following prolonged treatment. Vision, blurred Not known (see section 4.4) Respiratory, Epistaxis Very common Thoracic & Mediastinal disorders Nasal dryness, nasal irritation, throat Common dryness, throat irritation Nasal septal perforation Very rare Nasal ulcers Not known As with other nasal sprays, unpleasant taste and smell and headache have been reported. As with other nasal sprays, dryness and irritation of the nose and throat, and epistaxis have been reported. Nasal ulceration and nasal septal perforation have been reported following the use of intranasal corticosteroids, usually when there has been previous nasal surgery. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form: https://sideeffects.health.gov.il Additionally, please also report to GSK Israel (il.safety@gsk.com).

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
עלון מידע לצרכן
22.03.18 - עלון לצרכן 24.04.18 - עלון לצרכן 04.10.18 - עלון לצרכן אנגלית 04.10.18 - עלון לצרכן עברית 04.10.18 - עלון לצרכן ערבית 22.11.23 - עלון לצרכן עברית 23.02.24 - עלון לצרכן אנגלית 23.02.24 - עלון לצרכן עברית 23.02.24 - עלון לצרכן ערבית 12.04.24 - עלון לצרכן עברית 07.05.24 - עלון לצרכן אנגלית 07.05.24 - עלון לצרכן ערבית 01.08.24 - עלון לצרכן ערבית 28.11.23 - החמרה לעלוןלתרופה במאגר משרד הבריאות
פליקסונז תרסיס מימי לאף