Quest for the right Drug
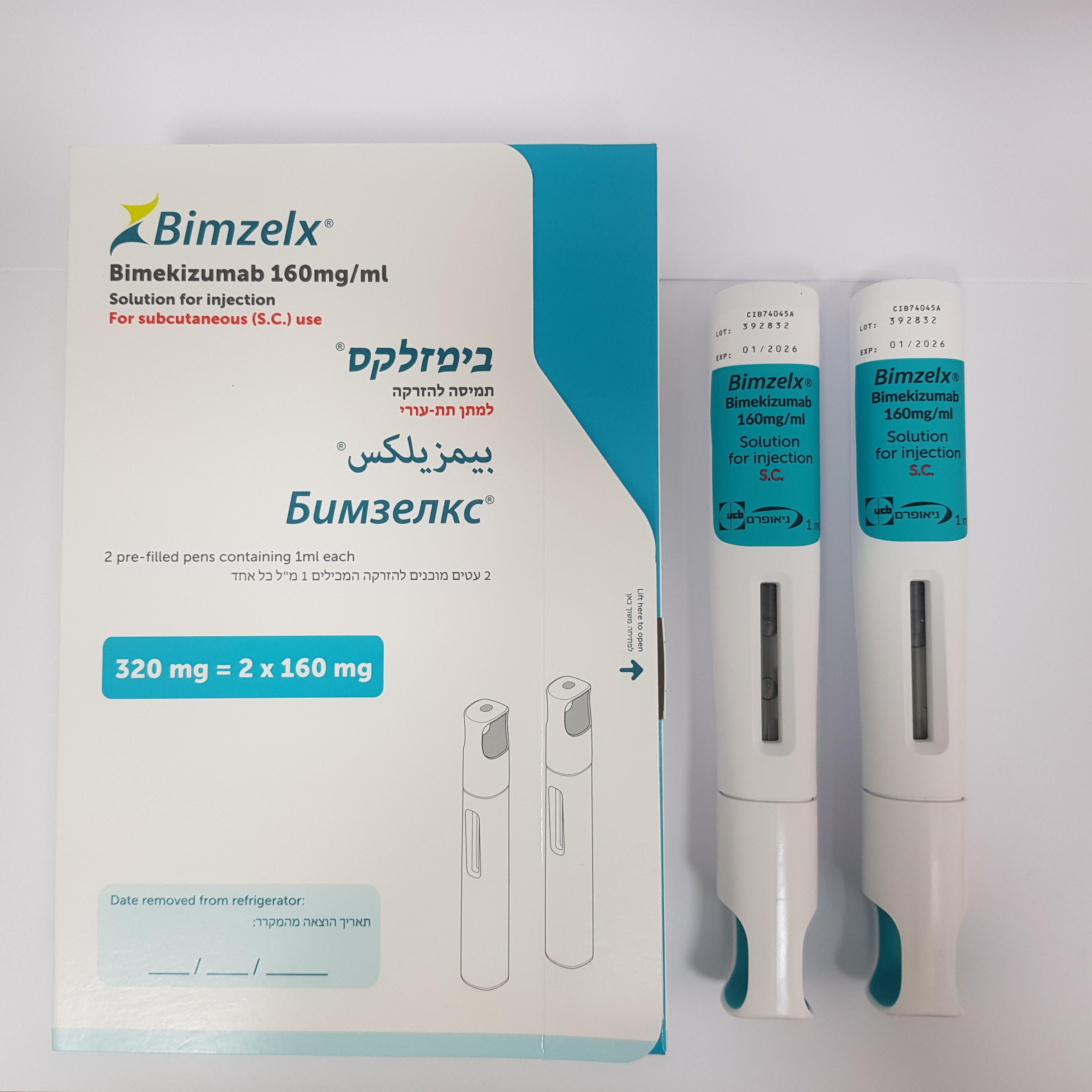
בימזלקס ® BIMZELX ® (BIMEKIZUMAB)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי : S.C
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects Summary of the safety profile The most frequently reported adverse reactions were upper respiratory tract infections (14.5%, 14.6%, 16.3% in plaque psoriasis, psoriatic arthritis and axial spondyloarthritis (axSpA) respectively) and oral candidiasis (7.3%, 2.3%, 3.7% in PSO, PsA and axSpA respectively). Tabulated list of adverse reactions Adverse reactions from clinical studies and post-marketing reports (Table 1) are classified by MedDRA System Organ Class and frequency, using the following convention: very common (≥ 1/10), common (≥ 1/100 to < 1/10), uncommon (≥ 1/1,000 to < 1/100), rare (≥ 1/10,000 to < 1/1,000), very rare (< 1/10,000), not known (cannot be estimated from the available data). Table 1: List of adverse reactions System Organ Class Frequency Adverse reaction Infections and infestations Very common Upper respiratory tract infections Common Oral candidiasis, Tinea infections, Ear infections, Herpes simplex infections, Oropharyngeal candidiasis, Gastroenteritis, Folliculitis, Vulvovaginal mycotic infection (including vulvovaginal candidiasis) Uncommon Mucosal and cutaneous candidiasis (including oesophageal candidiasis), Conjunctivitis Blood and lymphatic system Uncommon Neutropenia disorders Nervous System disorders Common Headache Gastrointestinal disorders Uncommon Inflammatory bowel disease Skin and subcutaneous tissue Common Rash, Dermatitis and eczema, disorders Acne General disorders and Common Injection site reactionsa, administration site conditions Fatigue a) Includes: injection site erythema, reaction, oedema, pain, swelling, haematoma. Description of selected adverse reactions Infections In the placebo-controlled period of Phase III clinical studies in plaque psoriasis, infections were reported in 36.0% of patients treated with bimekizumab for up to 16 weeks compared with 22.5% of patients treated with placebo. Serious infections occurred in 0.3% of patients treated with bimekizumab and 0% treated with placebo. The majority of infections consisted of non-serious mild to moderate upper respiratory tract infections such as nasopharyngitis. There were higher rates of oral and oropharyngeal candidiasis in patients treated with bimekizumab consistent with the mechanism of action (7.3% and 1.2% respectively compared to 0% for placebo-treated patients). More than 98% of cases were non-serious, mild or moderate in severity, and did not require treatment discontinuation. A slightly higher incidence of oral candidiasis was reported in patients <70 kg (8.5% versus 7.0% in patients ≥70 kg). Over the entire treatment period of Phase III studies in plaque psoriasis, infections were reported in 63.2% of patients treated with bimekizumab (120.4 per 100 patient-years). Serious infections were reported in 1.5% of patients treated with bimekizumab (1.6 per 100 patient-years) (see section 4.4). Infection rates observed in PsA and axSpA (nr-axSpA and AS) Phase III clinical studies were similar to those observed in plaque psoriasis apart from oral and oropharyngeal candidiasis rates in patients treated with bimekizumab, which were lower at 2.3% and 0% respectively in PsA and 3.7% and 0.3% respectively in axSpA compared to 0% with placebo. Neutropenia Neutropenia was observed with bimekizumab in phase III clinical studies in plaque psoriasis. Over the entire treatment period of Phase III studies, neutropenia grade 3/4 were observed in 1% of patients treated with bimekizumab. The frequency of neutropenia in PsA, axSpA (nr-axSpA and AS) clinical studies was similar to that observed in plaque psoriasis studies. Most cases were transient and did not require treatment discontinuation. No serious infections were associated with neutropenia. Hypersensitivity Serious hypersensitivity reactions including anaphylactic reactions have been observed with IL-17 inhibitors. Immunogenicity Plaque psoriasis Approximately 45% of plaque psoriasis patients treated with bimekizumab up to 56 weeks at the recommended dosing regimen (320 mg every 4 weeks up to week 16 and 320 mg every 8 weeks thereafter) developed anti-drug antibodies. Of the patients who developed anti-drug antibodies, approximately 34% (16% of all patients treated with bimekizumab) had antibodies that were classified as neutralising. Psoriatic arthritis Approximately 31% of patients with psoriatic arthritis treated with bimekizumab at the recommended dosing regimen (160 mg every 4 weeks) up to 16 weeks had anti-drug antibodies. Of the patients with anti-drug antibodies, about 33% (10% of all patients treated with bimekizumab) had antibodies that were classified as neutralising. By week 52, approximately 47% of biologic disease-modifying anti- rheumatic drug (bDMARD) treatment naïve patients with psoriatic arthritis in the BE OPTIMAL study treated with bimekizumab at the recommended dosing regimen (160 mg every 4 weeks) had anti-drug antibodies. Of the patients with anti-drug antibodies, about 38% (18% of all patients in the BE OPTIMAL study treated with bimekizumab) had antibodies that were classified as neutralising. Axial spondyloarthritis (nr-axSpA and AS) Approximately 57% of patients with nr-axSpA treated with bimekizumab up to 52 weeks at the recommended dosing regimen (160 mg every 4 weeks) had anti-drug antibodies. Of the patients with anti-drug antibodies, approximately 44% (25% of all patients treated with bimekizumab) had antibodies that were classified as neutralising. Approximately 44% of patients with AS treated with bimekizumab up to 52 weeks at the recommended dosing regimen (160 mg every 4 weeks) had anti-drug antibodies. Of the patients with anti-drug antibodies, approximately 44% (20% of all patients treated with bimekizumab) had antibodies that were classified as neutralising. Across indications, no clinically meaningful impact on clinical response was associated with anti- bimekizumab antibodies development and an association between immunogenicity and treatment emergent adverse events has not been clearly established. Elderly patients (≥65 years) Exposure is limited in elderly subjects. Elderly patients may be more likely to experience certain adverse reactions such as oral candidiasis, dermatitis and eczema when using bimekizumab. In the placebo-controlled period of Phase III clinical studies in plaque psoriasis, oral candidiasis was observed in 18.2% of patients ≥65 years versus 6.3 % in <65 years, dermatitis and eczema in 7.3% of patients ≥65 years versus 2.8% in <65 years. In the placebo-controlled period of Phase III clinical studies in psoriatic arthritis, oral candidiasis was observed in 7.0% of patients ≥65 years versus 1.6% in <65 years, dermatitis and eczema in 1.2% of patients ≥65 years versus 2.0% in <65 years. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il/ and emailed to the Registration Holder’s Patient Safety Unit at: drugsafety@neopharmgroup.com

פרטי מסגרת הכללה בסל
א. התרופה תינתן לטיפול בפסוריאזיס בהתקיים כל אלה: 1. החולה סובל מאחד מאלה: א. מחלה מפושטת מעל ל-50% של שטח גוף או PASI מעל 50; ב. נגעים באזורי גוף רגישים - אזורים אלו יכללו פנים, צוואר, קיפולי עור, כפות ידיים, כפות רגליים, אזור הגניטליה והישבן. 2. החולה קיבל שני טיפולים סיסטמיים לפחות ללא שיפור של 50% לפחות ב-PASI לאחר סיום הטיפול בהשוואה לתחילת הטיפול. בהתייחס לחולה העונה על פסקה (א)(1)(ב) החולה קיבל שני טיפולים סיסטמיים לפחות בלא שיפור משמעותי לאחר סיום הטיפול בהשוואה לתחילת הטיפול; ב. התרופה תינתן על פי מרשם של רופא מומחה בדרמטולוגיה.
מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| החולה קיבל שני טיפולים סיסטמיים לפחות ללא שיפור של 50% לפחות ב-PASI לאחר סיום הטיפול בהשוואה לתחילת הטיפול. התרופה תינתן על פי מרשם של רופא מומחה בדרמטולוגיה. | 17/03/2024 | עור ומין | פסוריאזיס | |
| התרופה תינתן לטיפול בפסוריאזיס בהתקיים כל אלה: 1. החולה סובל מאחד מאלה: א. מחלה מפושטת מעל ל-50% של שטח גוף או PASI מעל 50; ב. נגעים באזורי גוף רגישים - אזורים אלו יכללו פנים, צוואר, קיפולי עור, כפות ידיים, כפות רגליים, אזור הגניטליה והישבן. | 17/03/2024 | עור ומין | פסוריאזיס |
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
17/03/2024
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
עלון מידע לצרכן
07.06.23 - עלון לצרכן אנגלית 07.06.23 - עלון לצרכן עברית 07.06.23 - עלון לצרכן ערבית 09.11.23 - עלון לצרכן אנגלית 08.11.23 - עלון לצרכן עברית 09.11.23 - עלון לצרכן ערבית 20.09.24 - עלון לצרכן עברית 06.10.24 - עלון לצרכן עברית 25.11.24 - עלון לצרכן אנגלית 25.11.24 - עלון לצרכן ערבית 07.06.23 - החמרה לעלון 09.11.23 - החמרה לעלון 20.09.24 - החמרה לעלון 06.10.24 - החמרה לעלוןלתרופה במאגר משרד הבריאות
בימזלקס ®