Quest for the right Drug
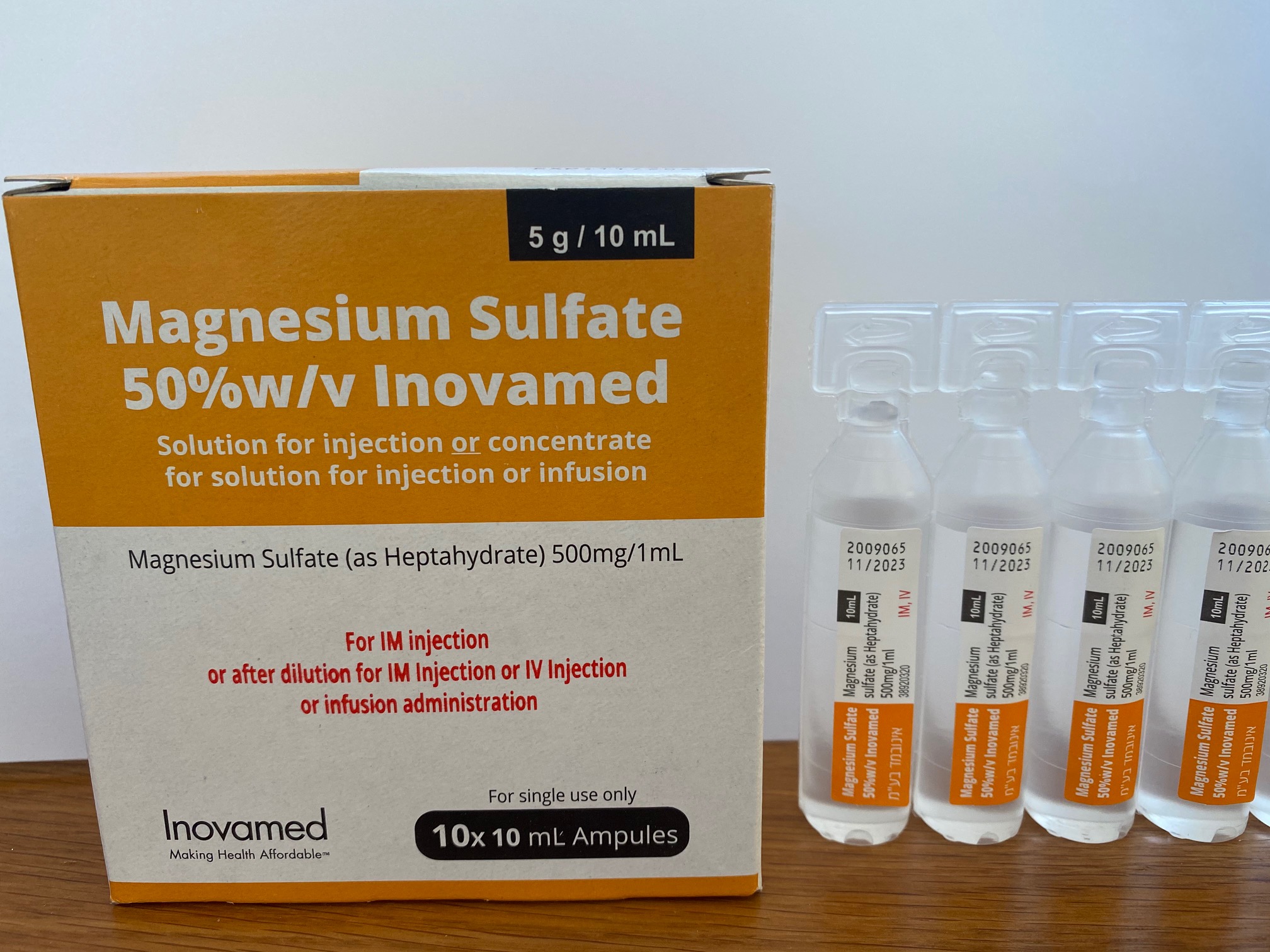
מגנזיום סולפאט 50%w/v אינובמד MAGNESIUM SULFATE 50 % W/V INOVAMED (MAGNESIUM SULFATE AS HEPTAHYDRATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי, תוך-שרירי : I.V, I.M
צורת מינון:
אין פרטים : SOLUTION FOR INJECTION / CONCENTRATE FOR SOLUTION FOR INJ/INF
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmaceutical particulars : מידע רוקחי
6. PHARMACEUTICAL PARTICULARS 6.1 List of excipients Water for injections Sulfuric acid (for pH adjustment) 6.2 Incompatibilities This medicinal product must not be mixed with other medicinal products/diluents except Glucose 5% or sodium chloride 0.9%. Magnesium sulfate is incompatible with alkali hydroxides (forming insoluble magnesium hydroxide), alkali carbonates (forming insoluble magnesium carbonate) and salicylates. The activities of streptomycin sulfate and tetramycin sulfate are inhibited by magnesium ions. 6.3 Shelf life The expiry date of the product is indicated on the packaging materials. After opening/dilution: Chemical and physical in-use stability has been demonstrated for 48 hours at 23 – 27°C and 2 – 8°C when diluted to a concentration of not more than 200 mg/mL magnesium sulfate heptahydrate in Sodium chloride 0.9% or Glucose 5%. From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibilities of the user and would normally not be longer than 24 hours at 2 – 8°C unless reconstitution/dilution (etc.) has taken place in controlled and validated aseptic conditions. 6.4 Special precautions for storage Do not store above 25oC . 6.5 Nature and contents of container Polypropylene ampoules of 10 mL containing a 50 % w/v solution of magnesium sulfate as heptahydrate for injection. Packed in cartons to contain 10, 20 or 50 ampoules. Not all pack sizes may be marketed. 6.6 Special precautions for disposal and other handling For intramuscular use, a 50% w/v solution is used. For intravenous use, the 50% w/v solution must be diluted before use, with a suitable diluent, such as Glucose 5% or sodium chloride 0.9%. Any unused medicinal product or waste material should be disposed of in accordance with local requirements. 7.MARKETING AUTHORIZATION HOLDER AND IMPORTER Inovamed Ltd. 55 Beer Ganim St. POB 62 Even Yehuda 40500

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
עלון מידע לרופא
25.01.21 - עלון לרופאעלון מידע לצרכן
לתרופה במאגר משרד הבריאות
מגנזיום סולפאט 50%w/v אינובמד