Quest for the right Drug
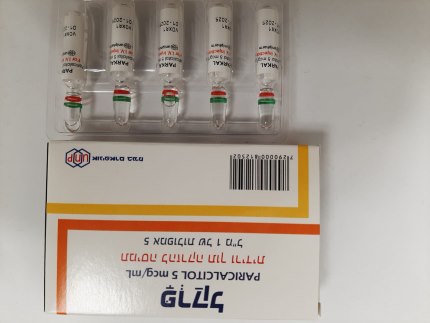
פרקל PARKAL (PARICALCITOL)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Overdose : מינון יתר
4.9 Overdose No case of overdose has been reported. Overdosage of paricalcitol may lead to hypercalcaemia, hypercalciuria, hyperphosphatemia, and over suppression of PTH (see section 4.4). In the event of an overdose, signs and symptoms of hypercalcemia (serum calcium levels) should be monitored and reported to a physician. Treatment should be initiated as appropriate. Paricalcitol is not significantly removed by dialysis. Treatment of patients with clinically significant hypercalcaemia consists of immediate dose reduction or interruption of paricalcitol therapy and includes a low calcium diet, withdrawal of calcium supplements, patient mobilisation, attention to fluid and electrolyte imbalances, assessment of electrocardiographic abnormalities (critical in patients receiving digitalis), and haemodialysis or peritoneal dialysis against a calcium-free dialysate, as warranted. When serum calcium levels have returned to within normal limits, paricalcitol may be reinitiated at a lower dose. If persistent and markedly elevated serum calcium levels occur, there are a variety of therapeutic alternatives that may be considered. These include the use of drugs such as phosphates and corticosteroids as well as measures to induce diuresis. Parkal solution for injection contains 38.6% v/v of propylene glycol as an excipient. Isolated cases of Central Nervous System depression, haemolysis and lactic acidosis have been reported as toxic effect associated with propylene glycol administration at high doses. Although they are not expected to be found with Parkal administration as propylene glycol is eliminated during the dialysis process, the risk of toxic effect in overdosing situations has to be taken into account.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
