Quest for the right Drug
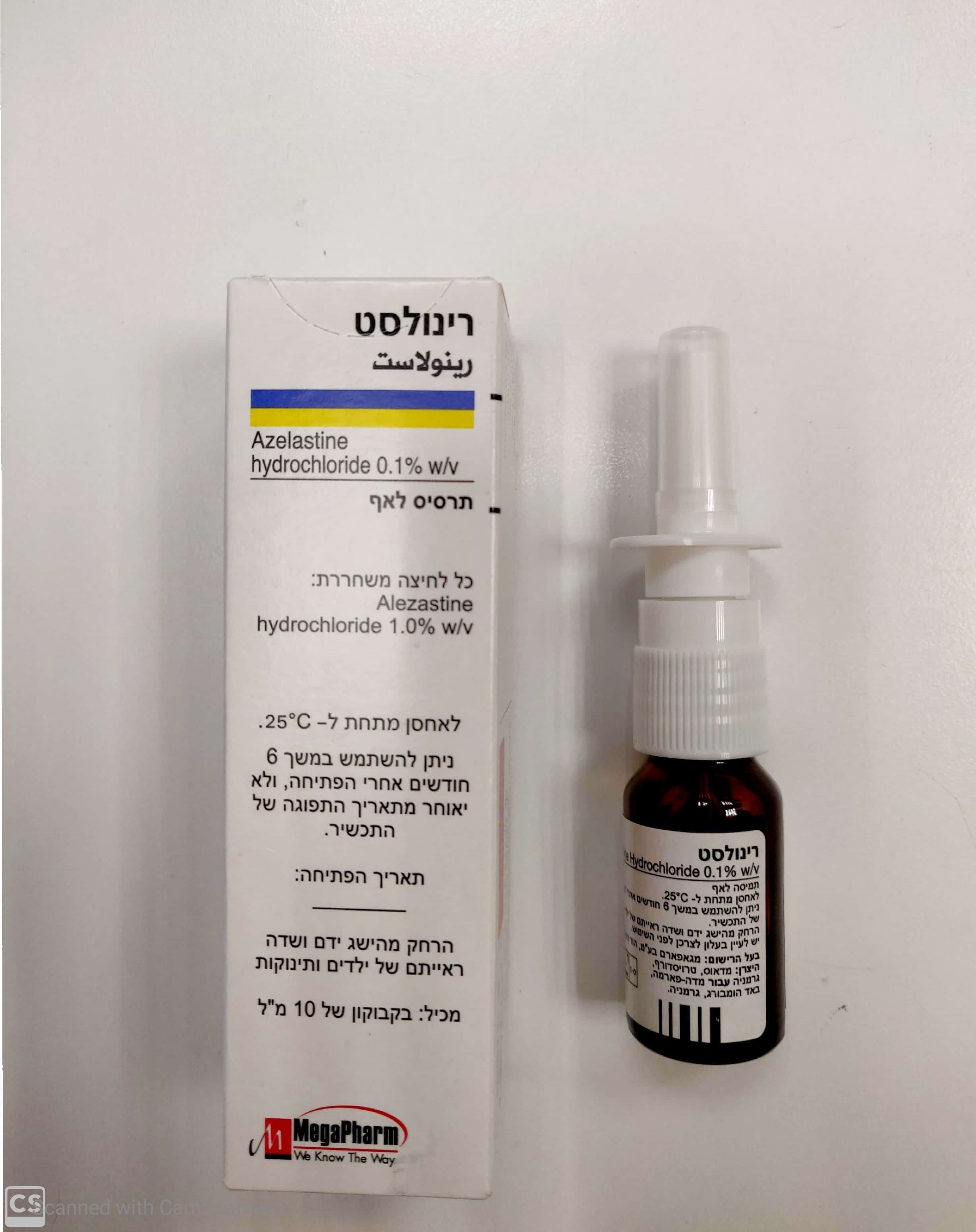
רינולסט RHINOLAST (AZELASTINE HYDROCHLORIDE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
אפי : NASAL
צורת מינון:
תמיסה לאף : NASAL SOLUTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1 Pharmacodynamic properties Azelastine, a phthalazinone derivative of novel structure, is classified as a potent long acting anti-allergic compound with particularly strong H1 antagonist properties. Data from animal studies show that when high levels of azelastine are achieved both inhibition and release of chemical mediators (e.g. leukotriene, histamine, serotonin) involved in allergic reaction occurs.
Pharmacokinetic Properties
5.2 Pharmacokinetic properties After repeated nasal application (0.14 mg) into each nostril twice daily, the plasma levels of azelastine were about 0.26 ng/ml. The levels of the active metabolite desmethylazelastine were detected at or below the lower limit of quantification (0.12 ng/ml). After repeated oral administration, the mean CMax steady state plasma levels were determined giving 3.9 ng/ml for azelastine and 1.86 ng/ml for desmethylazelastine after 2.2 mg b.i.d. azelastine which represents the therapeutic oral dose for the treatment of allergic rhinitis. Following oral administration azelastine is rapidly absorbed showing an absolute bioavailability of 81%. Food has no influence on absorption. The volume of distribution is high indicating distribution predominantly to the peripheral tissues. The level of protein binding is low (80-95%, a level too low to give concern over drug displacement reactions). Page 2 of 3 Plasma elimination half lives after a single dose of azelastine are approximately 20 hours for azelastine and about 45 hours for N- desmethylazelastine (a therapeutically active metabolite). Excretion occurs mainly via the faeces. The sustained excretion of small amounts of the dose in the faeces suggest that some enterohepatic circulation may take place.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
