Quest for the right Drug
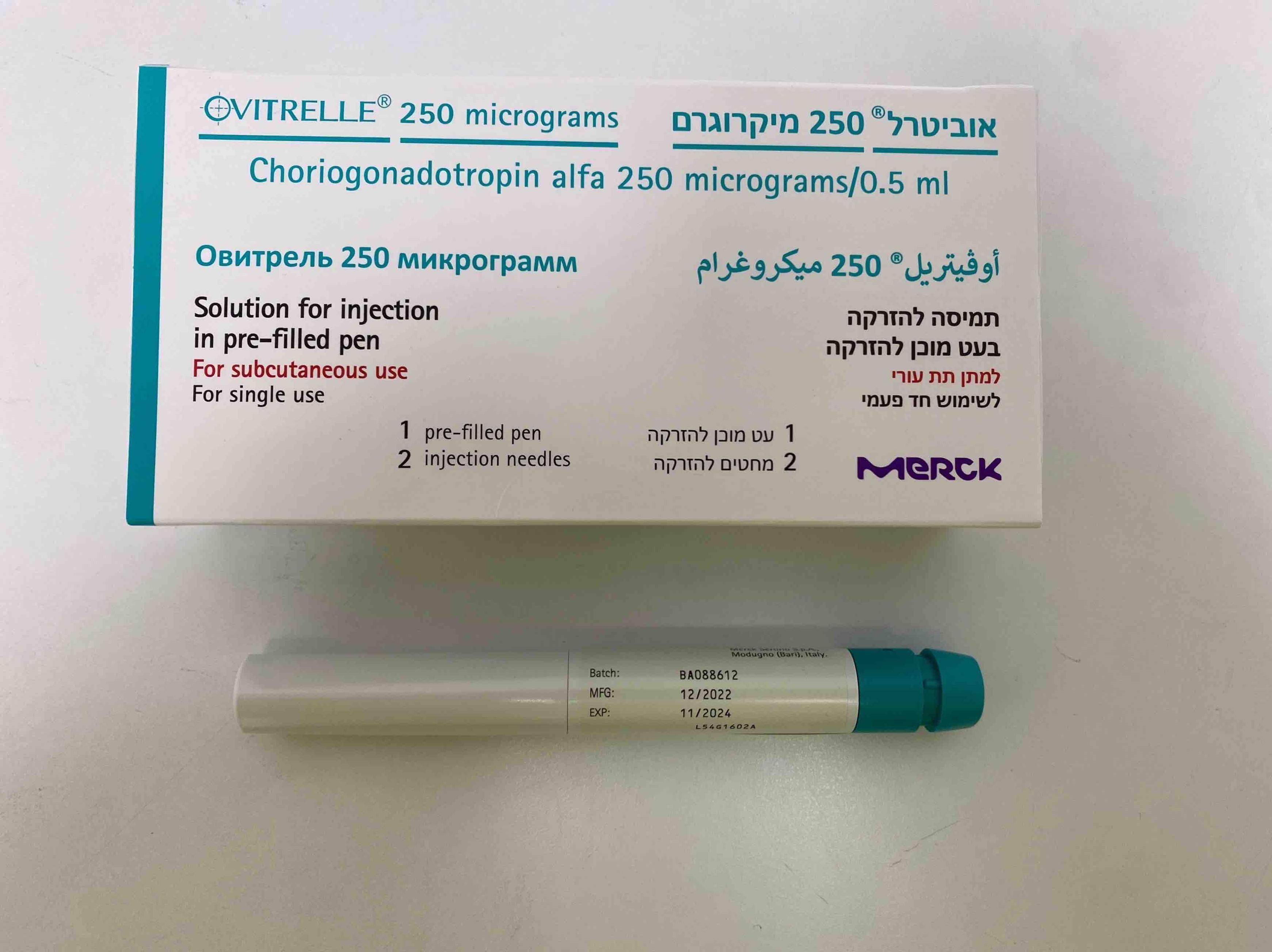
אוביטרל 250 מיקרוגרם OVITRELLE 250 MICROGRAMS (CHORIOGONADOTROPIN ALFA)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי : S.C
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use Traceability In order to improve the traceability of biological medicinal products, the name and the batch number of the administered product should be clearly recorded General precautions Before starting treatment, the couple's infertility should be assessed as appropriate and putative contraindications for pregnancy evaluated. In particular, patients should be evaluated for hypothyroidism, adrenocortical deficiency, hyperprolactinemia and pituitary or hypothalamic tumours, and appropriate specific treatment given. There is no clinical experience with Ovitrelle in the treatment of other conditions (such as corpus luteum insufficiency or male conditions) therefore Ovitrelle is not indicated in these conditions. Ovarian Hyperstimulation Syndrome (OHSS) A certain degree of ovarian enlargement is an expected effect of controlled ovarian stimulation. It is more commonly seen in women with polycystic ovarian syndrome and usually regresses without treatment. In distinction to uncomplicated ovarian enlargement, OHSS is a condition that can manifest itself with increasing degrees of severity. It comprises marked ovarian enlargement, high serum sex steroids, and an increase in vascular permeability which can result in an accumulation of fluid in the peritoneal, pleural and, rarely, in the pericardial cavities Mild manifestations of OHSS may include abdominal pain, abdominal discomfort and distension, and enlarged ovaries. Moderate OHSS may additionally present with nausea, vomiting, ultrasound evidence of ascites and marked ovarian enlargement. Severe OHSS further includes symptoms such as severe ovarian enlargement, weight gain, dyspnoea or oliguria. Clinical evaluation may reveal signs such as hypovolaemia, haemoconcentration, electrolyte imbalances, ascites, pleural effusions, or acute pulmonary distress. Very rarely, severe OHSS may be complicated by ovarian torsion or thromboembolic events, such as pulmonary embolism, ischaemic stroke or myocardial infarction. Independent risk factors for developing OHSS include young age, lean body mass, polycystic ovarian syndrome, higher doses of exogenous gonadotropins, high absolute or rapidly rising serum estradiol levels and previous episodes of OHSS, large number of developing ovarian follicles and large number of oocytes retrieved in ART cycles. Adherence to recommended Ovitrelle dosage and regimen of administration can minimise the risk of ovarian hyperstimulation. Monitoring of stimulation cycles by ultrasound scans as well as estradiol measurements are recommended to early identify risk factors. There is evidence to suggest that hCG plays a key role in triggering OHSS and that the syndrome may be more severe and more protracted if pregnancy occurs. Therefore, if signs of ovarian hyperstimulation occur, it is recommended that hCG be withheld and the patient be advised to refrain from coitus or use barrier contraceptive methods for at least 4 days. As OHSS may progress rapidly (within 24 hours) or over several days to become a serious medical event, patients should be followed for at least two weeks after hCG administration. Mild or moderate OHSS usually resolves spontaneously. If severe OHSS occurs, it is recommended that gonadotropin treatment be stopped and that the patient be hospitalised and appropriate therapy be started Multiple pregnancy In patients undergoing induction of ovulation, the incidence of multiple pregnancy and births is increased compared with natural conception. The majority of multiple conceptions are twins. Multiple pregnancies, especially high order, carry an increased risk of adverse maternal and perinatal outcomes. To minimise the risk of higher order multiple pregnancy, careful monitoring of ovarian response is recommended. In patients undergoing ART procedures the risk of multiple pregnancy is related mainly to the number of embryos replaced, their quality and the patient age. Pregnancy loss The incidence of pregnancy loss by miscarriage or abortion is higher in patients undergoing stimulation of follicular growth for ovulation induction or ART than following natural conception. Ectopic pregnancy Women with a history of tubal disease are at increased risk for ectopic pregnancy, whether the pregnancy is obtained by spontaneous conception or with fertility treatments. The prevalence of ectopic pregnancy after ART in this population was reported to be higher than in the general population. Congenital malformations The prevalence of congenital malformations after ART may be slightly higher than after spontaneous conceptions. This is thought to be due to differences in parental characteristics (e.g. maternal age, sperm characteristics) and the higher incidence of multiple pregnancies. Thromboembolic events In women with recent thromboembolic disease or women with generally recognised risk factors for thromboembolic events, such as personal or family history, treatment with gonadotropins may further increase the risk for aggravation or occurrence of such events. In these women, the benefits of gonadotropin administration need to be weighed against the risks. It should be noted, however, that pregnancy itself as well as OHSS also carry an increased risk of thromboembolic events. Reproductive system neoplasms There have been reports of ovarian and other reproductive system neoplasms, both benign and malignant, in women who have undergone multiple treatment regimens for infertility. It is not yet established whether or not treatment with gonadotropins increases the risk of these tumours in infertile women. Interference with serum or urinary testing Following administration, Ovitrelle may interfere for up to ten days with the immunological determination of serum or urinary hCG, potentially leading to a false positive pregnancy test. Patients should be made aware of this. Sodium content This medicinal product contains less than 1 mmol sodium (23 mg) per dose, i.e. it is essentially “sodium free”
Effects on Driving
4.7 Effects on ability to drive and use machines Ovitrelle has no or negligible influence on the ability to drive and use machines.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
עלון מידע לרופא
06.01.22 - עלון לרופאעלון מידע לצרכן
15.12.21 - עלון לצרכן אנגלית 15.12.21 - עלון לצרכן עברית 15.12.21 - עלון לצרכן ערבית 19.04.22 - עלון לצרכן אנגלית 19.04.22 - עלון לצרכן עברית 06.01.22 - עלון לצרכן ערבית 27.02.23 - עלון לצרכן עברית 23.08.23 - עלון לצרכן אנגלית 23.08.23 - עלון לצרכן עברית 23.08.23 - עלון לצרכן ערבית 12.04.16 - החמרה לעלון 12.09.18 - החמרה לעלון 25.12.18 - החמרה לעלון 27.07.21 - החמרה לעלון 06.01.22 - החמרה לעלון 27.02.23 - החמרה לעלוןלתרופה במאגר משרד הבריאות
אוביטרל 250 מיקרוגרם