Quest for the right Drug
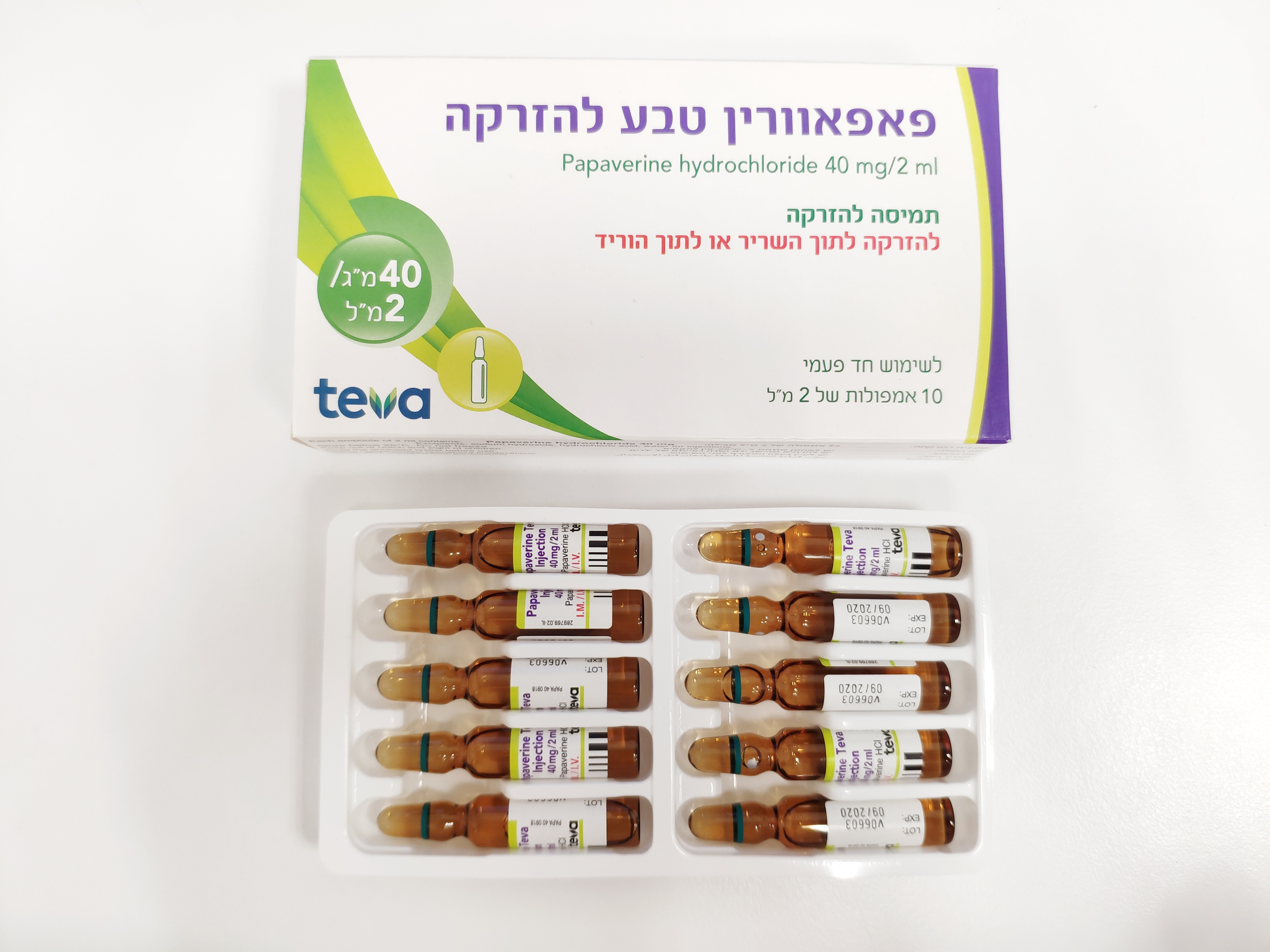
פאפאוורין טבע להזרקה PAPAVERINE TEVA INJECTION (PAPAVERINE HYDROCHLORIDE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-שרירי, תוך-ורידי : I.M, I.V
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmaceutical particulars : מידע רוקחי
6. PHARMACEUTICAL PARTICULARS 6.1 List of excipients Sodium hydroxide (for pH adjustment), Hydrochloric acid (for pH adjustment), Water for Injections. 6.2 Incompatibilities Do not add the papaverine solution to a lactated Ringer's solution as it may form a precipitate. 6.3 Shelf life The expiry date of the product is indicated on the packaging materials. 6.4 Special precautions for storage Store below 25°C. Do not freeze. 6.5 Nature and contents of container Carton box containing 10 amber glass ampoules. Each ampoule contains 2 ml solution. 6.6 Special precautions for disposal and handling Before administration, Papaverine Teva Injection should be drawn out according to the rules of good clinical practice, in the most aseptic manner possible, using a sterile syringe, immediately after opening the ampule. The drawn out drug solution should then be administered immediately. The solution should be visually inspected prior to administration for any particulate matter. Do not use the solution if the liquid is not clear. Discard ampules containing visible particles. This solution does not contain any antimicrobial preservative and is therefore for single use, which is not likely to prevent microorganism growth. Any unused medicinal product must not be stored for later use. Any unused product or waste material should be disposed of in accordance with the current regulations. In general, there is a risk of irritation or necrosis at the injection site in case of too rapid administration or if too much volume is injected. 7. LICENCE HOLDER AND MANUFACTURER TEVA ISRAEL LTD 124 Dvora HaNevi'a St., Tel Aviv 6944020 Israel

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
