Quest for the right Drug
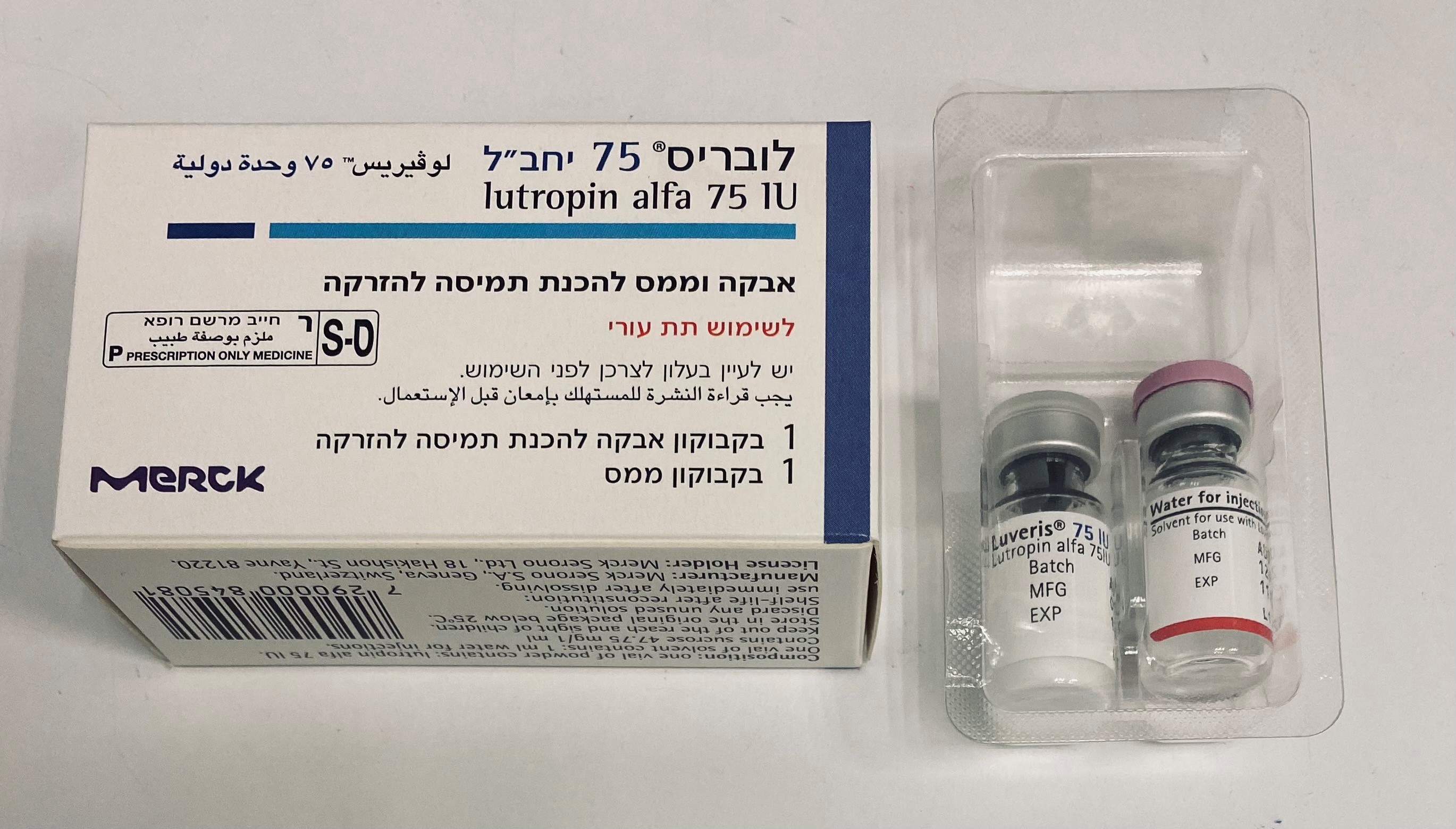
לובריס 75 LUVERIS 75 IU (LUTEINIZING HORMONE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי : S.C
צורת מינון:
אבקה להכנת תמיסה לזריקה : POWDER FOR SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1 Pharmacodynamic properties Pharmacotherapeutic group: Sex hormones and modulators of the genital system, gonadotropins. ATC code: G03GA07 Mechanism of action Luteinising hormone (LH) and follicle stimulating hormone (FSH) are secreted from the anterior pituitary gland in response to gonadotropin-releasing hormone (GnRH) and play a complementary role in follicle development and ovulation. In theca cells, LH stimulates the secretion of androgens that are transferred to granulosa cells to be converted to estradiol (E2) by aromatase. In granulosa cells, FSH stimulates the development of ovarian follicles, while LH action is involved in follicle development, steroidogenesis and maturation. Pharmacodynamic effects The primary effect resulting from administration of r-hLH is a dose-related increase of E2 secretion, enhancing the effect of FSH administration on follicular growth. Clinical efficacy In clinical trials, patients were defined by an endogenous serum LH level < 1.2 IU/L as measured in a central laboratory. In these trials the ovulation rate per cycle was 70 to 75%. However, it should be taken into account that there are variations between LH measurements performed in different laboratories. In one clinical study of women with hypogonadotropic hypogonadism and an endogenous serum LH concentration below 1.2 IU/L the appropriate dose of r-hLH was investigated. A dose of 75 IU r-hLH daily (in combination with 150 IU r-hFSH) resulted in adequate follicular development and estrogen production. A dose of 25 IU r-hLH daily (in combination with 150 IU r-hFSH) resulted in insufficient follicular development.
Pharmacokinetic Properties
5.2 Pharmacokinetic properties The pharmacokinetics of lutropin alfa have been studied in pituitary desensitised female volunteers from 75 IU up to 40,000 IU. The pharmacokinetic profile of lutropin alfa is similar to that of endogenous LH. There is no pharmacokinetic interaction with follitropin alfa when administered simultaneously. Distribution Following intravenous administration, lutropin alfa is rapidly distributed with an initial half-life of approximately one hour and eliminated from the body with a terminal half-life of about 9 to 11 hours. The steady state volume of distribution is in the range of 5 to 14 L. Lutropin alfa shows linear pharmacokinetics, as assessed by area under curve (AUC) which is directly proportional to the dose administered. Following subcutaneous administration, the absolute bioavailability is 56% and the apparent terminal half-life is in the range of 8 to 21 hours. Dose proportionality after subcutaneous administration was demonstrated up to 450 IU. The lutropin alfa pharmacokinetics following single and repeated administration of Luveris are comparable and the accumulation ratio of lutropin alfa is minimal. Elimination Total body clearance is around 1.8 L/h and less than 5% of the dose is excreted in the urine.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
