Quest for the right Drug
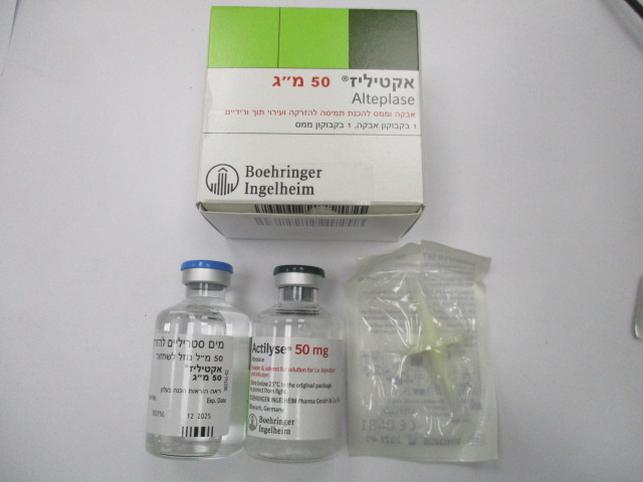
אקטיליז 50 מ"ג ACTILYSE 50 MG (ALTEPLASE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
אבקה וממס להכנת תמיסה להזרקהאינפוזיה : POWDER AND SOLVENT FOR SOLUTION FOR INJECTION/INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Contraindications : התוויות נגד
4.3 Contraindications Hypersensitivity to the active substance alteplase, gentamicin (a trace residue from the manufacturing process) or to any of the excipients listed in section 6.1. Contraindications in acute myocardial infarction, acute massive pulmonary embolism and acute ischaemic stroke: Actilyse is contraindicated in cases where there is a high risk of haemorrhage such as: significant bleeding disorder at present or within the past 6 months known haemorrhagic diathesis • patients receiving effective oral anticoagulant treatment (e.g. warfarin sodium with INR >1.3) (see section 4.4) manifest or recent severe or dangerous bleeding known history of or suspected intracranial haemorrhage suspected subarachnoid haemorrhage or condition after subarachnoid haemorrhage from aneurysm any history of central nervous system damage (i.e. neoplasm, aneurysm, intracranial or spinal surgery) recent (less than 10 days) traumatic external heart massage, obstetrical delivery, recent puncture of a non-compressible blood-vessel (e.g. subclavian or jugular vein puncture) severe uncontrolled arterial hypertension bacterial endocarditis, pericarditis acute pancreatitis documented ulcerative gastrointestinal disease during the last 3 months, oesophageal varices, arterial-aneurysm, arterial/venous malformations neoplasm with increased bleeding risk severe liver disease, including hepatic failure, cirrhosis, portal hypertension (oesophageal varices) and active hepatitis major surgery or significant trauma in past 3 months. Additional contraindications in acute myocardial infarction: any known history of haemorrhagic stroke or stroke of unknown origin . known history of ischaemic stroke or transient ischaemic attack (TIA) in the preceding 6 months, except current acute ischaemic stroke within 4.5 hours. Actilyse Updated Prescribing Information 20 mg, 50 mg Feb 2023 Additional contraindications in acute massive pulmonary embolism: any known history of haemorrhagic stroke or stroke of unknown origin. known history of ischaemic stroke or transient ischaemic attack (TIA) in the preceding 6 months, except current acute ischaemic stroke within 4.5 hours. Additional contraindications in acute ischaemic stroke: symptoms of ischaemic attack beginning more than 4.5 hours prior to infusion start or symptoms for which the onset time is unknown and could potentially be more than 4.5 hours ago (see section 5.1) minor neurological deficit or symptoms rapidly improving before start of infusion severe stroke as assessed clinically (e.g. NIHSS>25) and/or by appropriate imaging techniques seizure at onset of stroke evidence of intracranial haemorrhage (ICH) on the CT-scan symptoms suggestive of subarachnoid haemorrhage, even if CT-scan is normal administration of heparin within the previous 48 hours and a thromboplastin time exceeding the upper limit of normal for laboratory patients with any history of prior stroke and concomitant diabetes prior stroke within the last 3 months platelet count of below 100,000/mm3 systolic blood pressure > 185 mm Hg or diastolic BP > 110 mm Hg, or aggressive management (intravenous pharmacotherapy) necessary to reduce BP to these limits blood glucose < 50 mg/dl or > 400 mg/dl (<2.8mM or >22.2mM). Use in children and adolescents Actilyse is not indicated for the treatment of acute ischaemic stroke in children under 16 years of age (for adolescents ≥ 16 years of age see section 4.4).

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
